Abstract
Keywords
Propolis, Essential Oil Methanolic Extract Colorectal Cancer HCT116, Anticancer
1. Background
Colorectal cancer (CRC) is the fourth life-threatening cancer in the world, which is caused by the uncontrollable division and abnormal growth of the colorectal cells (1-3). Like all other types of cancer, surgical resection, chemotherapy, and radiotherapy are the main treatment options in clinical practice (4, 5). Despite the current therapies, there are still many limitations in the diagnosis and treatment of CRC patients. Today, the interest in developing new cancer therapy approaches based on the combination of conventional and unconventional therapy methods has increased. One of the most effective strategies for cancer prevention is ‘chemoprevention’ (6). According to the World Health Organization (WHO) reports, about 60% of drugs approved by the Food and Drug Administration (FDA) in the 1990s had been derived from natural and plant products (7). Accordingly, the discovery and development of new materials in cancer therapy is essential.
Propolis is a natural material that is mainly collected by honey bees from the bark and leaf buds of various plant species to build and repair the hives (8, 9). It is composed of vegetable balsam and resin, aromatic and essential oils, pollen, wax, and other substances (10). Due to its toxicity, propolis protects the hive against invading larvae, fungi, and bacteria (11). Propolis is found in different geographic regions such as temperate and tropical climates and has long been used as a remedy in folk medicine (12). Previous studies have been reported that the chemical compositions of propolis are different in various geographic regions due to the various plant sources (13, 14). In addition to the beneficial properties of propolis for bees, this natural material has pharmacological values as well (13, 15). Terpenoids and aromatic carboxylic acids are the dominant components of propolis essential oil. Terpenoids of propolis have several pharmacological and biological activities, such as antibacterial, antioxidant, antitumor, and anti-inflammatory effects (16). Propolis flavonoids have also exhibited an anticancer effect both in vivo and in vitro (17). In addition to flavonoids, other anticancer contents of propolis include terpenes and caffeic acid phenethyl ester (18). Several studies have demonstrated that propolis plays an effective anticancer activity for some types of cancers, including prostate cancer (19), breast cancer (20), bladder carcinoma, and hepatic carcinoma (21).
2. Objectives
Since propolis of various bee species, geographical regions, and seasons have different chemical properties, in this study, we investigated the effects of essential oil and methanolic extract of Hamadan propolis on CRC cell line and assessed its chemical composition.
3. Methods
3.1. Cell Line and Culture Conditions
Human colorectal adenocarcinoma cell line HCT116 was purchased from the Pasteur Institute (Tehran, Iran) and cultured in high glucose DMEM medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% antibiotic (penicillin/streptomycin). HCT116 cells were incubated at 37°C with 5% CO2 humid air.
3.2. Propolis Preparation
Propolis was collected in June 2017 from Hamadan City in Iran and stored in the dark place at 4°C until used.
3.3. Preparation of the Essential Oil
The essential oil of Hamadan propolis was extracted from 120 g propolis powder in three hours through the hydrodistillation method and by Clevenger-type device (22). Finally, the collected essential oil was dehydrated by anhydrous sodium sulfate.
3.4. Preparation of Methanolic Extract
To prepare the methanolic extract, 100 g of propolis was extracted by methanol through the maceration method (3 × 1 L, rt for 72 hours) (23). The extract was concentrated using the rotary evaporator systems to generate a dark gummy solid.
3.5. MTT Assay
The antiproliferative effect of Hamadan propolis essential oil and the methanolic extract was evaluated using the 3-(4, 5-dimethyl -2-thiazolyl) -2, 5-diphenyl -2H-tetrazolium bromide (MTT) assay. Briefly, HCT116 cells were seeded in 96-well plates (7000 cells/well). These cells were incubated with different concentrations of Hamadan propolis essential oil (0, 4, 8, 16, 32, and 64 µg/mL) and methanolic extract (0, 70, 80.5, 92.5, 106.4, 122, 140, 161, 186, and 214 µg/mL). After addition of MTT solution to each well and incubation for 3 hours, the mixture was discarded, and 100 µL dimethyl sulfoxide (DMSO) was used to dissolve the formazan crystals. The wavelength of 570 nm was used to measure the optical density (OD). All results in MTT assays were repeated three times, and IC50 (inhibitory concentration 50%) was calculated according to our previous study (24).
3.6. Identification of Essential Oil Compounds
The composition of the Hamadan propolis essential oil was characterized by retention indices (RI) and comparing their mass spectra with those of the related gas chromatography-mass spectrometry (GC-MS) and GC library. GC analysis was performed using a Thermoquest gas chromatograph with a detector of flame ionization (FID) on DB-5 GC column (60 m, 0.25 mm, 0.25 µm). The 250°C and 300°C temperatures were regulated respectively for the injector and detector. The oven temperature was regulated from 60°C to 250°C at the rate of 5°C/min and ultimately held isothermally for 2 minutes. GC-MS analysis was carried out the same as GC column by a gas chromatograph connected to a TRACE mass spectrometer. Moreover, the carrier gas used was Helium, and Ionization voltage was 70 ev. The 250°C and 200°C temperatures were regulated respectively for the interface and ion source. Mass range from 43 to 456 m/z was scanned (25).
3.7. Determination of Total Phenolic and Flavonoid Content
The total flavonoid and phenolic content of Hamadan propolis methanolic extract were assessed using the aluminum chloride colorimetric procedures and Folin-Ciocalteu (F-C) assay (26, 27), respectively, according to the protocol of Kerdar et al. (28).
4. Results
4.1. Anticancer Activity Effects of Hamadan Propolis
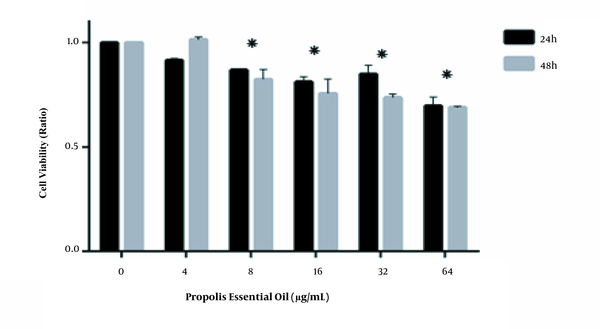
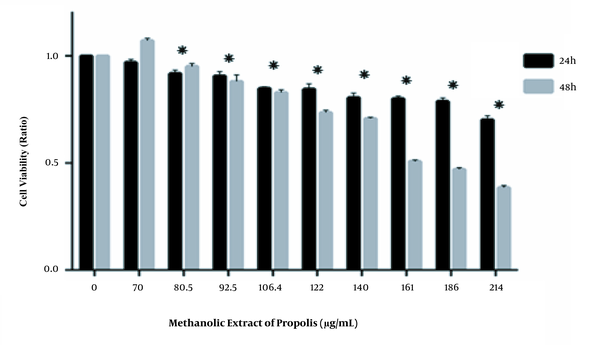
We investigated the effect of various concentrations of Hamadan propolis essential oil (0, 4, 8, 16, 32, and 64 µg/mL) and methanolic extract (0, 70, 80.5, 92.5, 106.4, 122, 140, 161, 186, and 214 µg/mL) on HCT116 cells viability by MTT assay. As illustrated in Figures 1 and 2, Hamadan propolis essential oil and methanolic extract decreased the percentage of viable cells compared with the untreated cell in a time- and concentration-dependent pattern. Hamadan propolis essential oil and methanolic extract displayed significant cytotoxicity at 8 and 80.5 μM, respectively (in 24 and 48 hours, P < 0.05). The 50% inhibition concentration (IC50) values were 356.7 and 296.4 µg/mL after 24 and 48 hours for essential oil and 702.5 and 177.7 µg/mL after 24 and 48 hours for methanolic extract.
Effects of the propolis essential oil on (HCT-116) Cell Viability. Propolis essential oil doses equal to or greater than 8 µg/mL led to a significant decrease in cell viability after 24 and 48 hours (*, P-value < 0.05).
Effects of the methanolic extract of hamadan propolis on (HCT-116) Cell Viability. Doses equal to or greater than 80.5 µg/mL of methanolic extract led to a significant decrease in cell viability after 24 and 48 hours (*, P-value < 0.05).
4.2. The Compounds of Hamadan Propolis Essential Oil
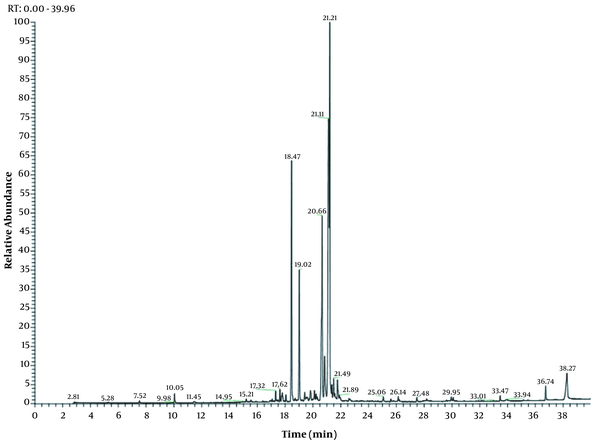
Hydrodistillation of Hamadan propolis provided a light yellow color essential oil between yield and w/w. The volatile compounds of propolis were identified. The percentage and retention index profiles are listed in Table 1. A total of 30 compounds were characterized, comprising 95.7% of the volatile oil. The major compounds of the essential oil were identified as β-u desmol (25.7%), α-u desmol (20.4%), α-copaen-11-o1 (13.7%), and γ-u desmol (10.1%) (Table 1 and Figure 3).
| Number | RT | Area, % | RIA | RIB | Compound | Method of Identification |
|---|---|---|---|---|---|---|
| 1 | 7.52 | 0.1 | 1104 | 1100 | n-Nonanal | RI, MS |
| 2 | 10.05 | 0.4 | 1207 | 1201 | n-Decanal | RI, MS |
| 3 | 15.21 | 0.1 | 1408 | 1408 | Dodecanal | RI, MS |
| 4 | 15.54 | 0.1 | 1421 | 1417 | (E)-Caryophyllene | RI, MS |
| 5 | 17.07 | 0.1 | 1484 | 1479 | ar-Curcumene | RI, MS |
| 6 | 17.32 | 0.5 | 1494 | 1491 | 10,11-epoxy-Calamenene | RI, MS |
| 7 | 17.4 | 0.1 | 1497 | 1492 | cis-β-Guaiene | RI, MS |
| 8 | 17.51 | 0.1 | 1502 | 1500 | α-Muurrolene | RI, MS |
| 9 | 17.8 | 0.8 | 1514 | 1514 | Butylated hydroxytuloene | RI, MS |
| 10 | 18.06 | 0.3 | 1525 | 1522 | δ-Cadinene | RI, MS |
| 11 | 18.47 | 13.7 | 1543 | 1539 | α-Copaen-11-ol | RI, MS |
| 12 | 18.72 | 0.2 | 1554 | 1548 | Elemol | RI, MS |
| 13 | 19.02 | 7.0 | 1567 | 1561 | (E)-Nerolidol | RI, MS |
| 14 | 19.41 | 0.5 | 1583 | 1577 | Spathulenol | RI, MS |
| 15 | 19.82 | 0.9 | 1601 | 1600 | Guaiol | RI, MS |
| 16 | 20.1 | 0.6 | 1613 | 1608 | β-Atlantol | RI, MS |
| 17 | 20.23 | 0.5 | 1619 | 1618 | 1,10-di-epi-Cubenol | RI, MS |
| 18 | 20.62 | 3.7 | 1637 | 1629 | Eremoligenol | RI, MS |
| 19 | 20.66 | 10.1 | 1639 | 1630 | γ-Eudesmol | RI, MS |
| 20 | 20.84 | 3.5 | 1647 | 1640 | Hinesol | RI, MS |
| 21 | 21.11 | 25.7 | 1659 | 1649 | β-Eudesmol | RI, MS |
| 22 | 21.21 | 20.4 | 1663 | 1652 | α-Eudesmol | RI, MS |
| 23 | 21.57 | 0.3 | 1679 | 1666 | 14-hydroxy-(Z)-Caryophyllene | RI, MS |
| 24 | 21.77 | 1.2 | 1688 | 1685 | α-Bisabolol | RI, MS |
| 25 | 21.9 | 0.4 | 1694 | 1685 | Germacra-4(15),5,10(14)-trien-1α-ol | RI, MS |
| 26 | 26.14 | 0.2 | 1897 | 1900 | n-Nonadecane | RI, MS |
| 27 | 29.95 | 0.2 | 2101 | 2100 | Heneicosane | RI, MS |
| 28 | 33.47 | 0.3 | 2299 | 2300 | Tricosane | RI, MS |
| 29 | 36.74 | 0.7 | 2500 | Pentacosane | MS | |
| 30 | 38.27 | 3.4 | 2800 | Octacosane | MS |
GC-Mass chromatogram of Hamadan propolis essential oil
4.3. The Contents of Total Phenolic and Flavonoid
Evaluation of phenolic and flavonoid components of methanolic extracts of Hamadan propolis indicated that this extract consists of 32.01 mg/g dry weight total phenolic and 14.12 mg/g dry weight total flavonoid content.
5. Discussion
Propolis and its derivatives have beneficial effects in the treatment of cancer due to the strong antitumor properties (29). Propolis has previously been shown to be constructive in reducing recurrent CRC, but Hamadan methanolic extract and essential oil effects are unknown. Various propolis species and their active compounds, such as caffeic acid phenethyl ester (CAPE) and chrysin have been used in modern and traditional medicine due to their anticancer properties (30).
The purpose of this study was to determine the essential oil and methanolic extract effects of Hamadan propolis upon the growth and proliferation of the HCT116 colon cancer cell line. Hence, we first prepared propolis essential oil and methanolic extract from Hamadan plant species. Next, the HCT116 colon cancer cell line was treated with diverse concentrations of propolis essential oil and methanolic extract, and their cytotoxicity was evaluated using MTT assay. Finally, the chemical compositions and total phenolic and flavonoid content of Hamadan propolis were analyzed.
The major identified compounds of the essential oil included β-u desmol (25.7%), α-u desmol (20.4%), α-copaen-11-o1 (13.7%), and γ-u desmol (10.1%) (Table 1 and Figure 3). The chemical compound of propolis analyzed in the present study was different from that collected in Brazil; the main composition of Brazilian propolis essential oil included prenylated acetophenones spathulenol, benzyl benzoate, and (2Z, bE)-farnesol (31). Also, from the 26 identified constituents of the other study in Brazilian propolis, β-caryophyllene, β-farnesene, and acetophenone were found to be the major components (15). Results of another study indicated that 17 compounds make up 91.0% of compounds present in the oil, and the main components were the β-caryophyllene, diene, selina-3,7, and (E) nerolidol (32). These results showed that these compounds are different in different geographical regions, even inside a country.
One of the most important constituents of Hamadan propolis was β-u desmol. This is in line with some previous studies from Hungary, France, Bulgaria, Brazil, and Northern Italy, which reported various healing properties such as antibacterial properties (33-36). Also, we identified other constituents such as α-eudesmol, α-copaen, and γ-u desmol in Hamadan propolis essential oil. In a survey by Vassya Bankova et al., α-eudesmol with 6.9% was identified as an important compound in Brazilian propolis (37). Similarly, in the studies by Oliveira et al. (15) and de Albuquerque et al. (32) on Brazilian propolis, α-copaen was identified with 3.03% and 4%, respectively.
Hamadan propolis methanolic extract consists of 32.01 mg/g dry weight total phenolic and 14.12 mg/g dry weight total flavonoid content. The methanolic extract of propolis was able to markedly affect the viability of the HCT116 cell line. The effect of anticancer activity of Hamadan propolis on HCT116 is thought to be due to their phenolic and flavonoid content. Studies have indicated that polyphenolic compounds derived from natural products have various beneficial properties, such as antitumor and antioxidant effects (38, 39). One of the phenolic compounds, which is also known as the active constituent in honeybee propolis, is CAPE. The anticancer properties of this compound have been confirmed in several studies (40-42). Consistent with previous studies, our in vitro results exhibited that the operation of methanolic extract of Hamadan propolis led to cellular morphological changes, especially antiproliferative and cytotoxic effects in the CRC cell lines, which can be mainly due to the presence of high phenolic materials such as CAPE.
Since the treatments based on the essential oil of propolis have not been reported previously, we assumed that it can be effective in interfering with the growth, proliferation, and differentiation in cancer cells due to its toxicity. The HCT116 cells were treated with different concentrations of propolis essential oil. The essential oil decreased HCT116 cells viability in a time- and concentration-dependent pattern after 24 and 48 hours of treatment.
Also, our in-vitro results exhibited that the operation of the Hamadan propolis methanolic extract led to cellular morphological changes, especially antiproliferative and cytotoxic effects in the HCT116 cell line. Consistent with this study, Kustiawan et al. (43) showed cytotoxic activity of Indonesia-Trigona incisa ethanolic extract of propolis (EEP) against the SW620 CRC cell lines with 8% of survival at 20 µg/mL of drug. Also, Salim et al. reported cytotoxic activity of EEP against PC3 cell line of prostate cancer in a dose-dependent manner (19). Kubina et al. (44) in Poland demonstrated that ethanol extract of propolis had antineoplastic effects with the stimulation of the apoptosis process in different carcinoma cells, including the HCT116 colon cancer cell line and ME45 malignant melanoma cell line. Drigla et al. (45) evaluated the effect of propolis aqueous extract on breast cancer cell lines. Their results indicated that propolis extract has antitumor effects on both cell lines.
In another study, Oliveira et al. (15) exhibited antibacterial effects of essential oil of propolis against Escherichia coli (E. coli), Streptococcus pyogenes (S. pyogenes), Staphylococcus aureus (S. aureus), and Staphylococcus epidermidis (S. epidermidis). The results of Li et al. (37) showed the therapeutic effects of essential oil from Brazilian green propolis on anxiety (as neurodegenerative diseases) by improving the antioxidation capability on brain tissue and affecting the hypothalamic-pituitary-adrenal (HPA) axis.
Although few studies have been performed on the antitumor properties of propolis, it is thought to have antitumor properties. In this regard, Abikim et al. (46) demonstrated the inhibitory activity of Chinese propolis essential oils against the proliferation of CRC cells through promoting cell apoptosis and cycle arrest.
5.1. Conclusions
The most important constituents of Hamadan propolis essential oil were β-u desmol, α-eudesmol, α-copaen, and γ-u desmol. The results of the current study suggested that the essential oil and methanolic extract of Hamadan propolis have anticancer activity on HCT116. Finally, we suggest further evaluation of the cytotoxic effects of the most important constituents of propolis essential oil and major compounds of propolis methanolic extract.
References
-
1.
Afshar S, Afshar S, Warden E, Manochehri H, Saidijam M. Application of Artificial Neural Network in miRNA Biomarker Selection and Precise Diagnosis of Colorectal Cancer. Iran Biomed J. 2019;23(3):175-83. [PubMed ID: 30056689]. [PubMed Central ID: PMC6462295].
-
2.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-91. [PubMed ID: 26818619]. https://doi.org/10.1136/gutjnl-2015-310912.
-
3.
Tanzadehpanah H, Mahaki H, Moradi M, Afshar S, Rajabi O, Najafi R, et al. Human serum albumin binding and synergistic effects of gefitinib in combination with regorafenib on colorectal cancer cell lines. Colorectal Cancer. 2018;7(2). https://doi.org/10.2217/crc-2017-0018.
-
4.
Afshar S, Sedighi Pashaki A, Najafi R, Nikzad S, Amini R, Shabab N, et al. Cross-Resistance of Acquired Radioresistant Colorectal Cancer Cell Line to gefitinib and regorafenib. Iran J Med Sci. 2020;45(1):50-8. [PubMed ID: 32038059]. [PubMed Central ID: PMC6983275]. https://doi.org/10.30476/ijms.2019.44972.
-
5.
Mishra J, Drummond J, Quazi SH, Karanki SS, Shaw JJ, Chen B, et al. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol. 2013;86(3):232-50. [PubMed ID: 23098684]. [PubMed Central ID: PMC3561496]. https://doi.org/10.1016/j.critrevonc.2012.09.014.
-
6.
Wang J, Liu L, Qiu H, Zhang X, Guo W, Chen W, et al. Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells. PLoS One. 2013;8(5). e63872. [PubMed ID: 23737956]. [PubMed Central ID: PMC3667855]. https://doi.org/10.1371/journal.pone.0063872.
-
7.
Naveen Kumar DR, Cijo George V, Suresh PK, Ashok Kumar R. Cytotoxicity, apoptosis induction and anti-metastatic potential of Oroxylum indicum in human breast cancer cells. Asian Pac J Cancer Prev. 2012;13(6):2729-34. [PubMed ID: 22938449]. https://doi.org/10.7314/apjcp.2012.13.6.2729.
-
8.
Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73 Suppl 1:S1-6. [PubMed ID: 12495704]. https://doi.org/10.1016/s0367-326x(02)00185-5.
-
9.
Greenaway W, May J, Scaysbrook T, Whatley FR. Identification by Gas Chromatography-Mass Spectrometry of 150 Compounds in Propolis. Zeitschrift für Naturforschung C. 1991;46(1-2):111-21. https://doi.org/10.1515/znc-1991-1-218.
-
10.
Hwu YJ, Lin FY. Effectiveness of propolis on oral health: a meta-analysis. J Nurs Res. 2014;22(4):221-9. [PubMed ID: 25386865]. https://doi.org/10.1097/jnr.0000000000000054.
-
11.
Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64(3):235-40. [PubMed ID: 10363838]. https://doi.org/10.1016/s0378-8741(98)00131-7.
-
12.
Ghisalberti EL. Propolis: A Review. Bee World. 2015;60(2):59-84. https://doi.org/10.1080/0005772x.1979.11097738.
-
13.
Jafarzadeh Kashi TS, Kasra Kermanshahi R, Erfan M, Vahid Dastjerdi E, Rezaei Y, Tabatabaei FS. Evaluating the In-vitro Antibacterial Effect of Iranian Propolis on Oral Microorganisms. Iran J Pharm Res. 2011;10(2):363-8. [PubMed ID: 24250366]. [PubMed Central ID: PMC3828924].
-
14.
Markham KR, Mitchell KA, Wilkins AL, Daldy JA, Lu Y. HPLC and GC-MS identification of the major organic constituents in New Zeland propolis. Phytochemistry. 1996;42(1):205-11. https://doi.org/10.1016/0031-9422(96)83286-9.
-
15.
Oliveira AP, França HS, Kuster RM, Teixeira LA, Rocha LM. Chemical composition and antibacterial activity of Brazilian propolis essential oil. J Venomous Anim Toxins includ Trop Dis. 2010;16(1):121-30. https://doi.org/10.1590/s1678-91992010005000007.
-
16.
de las Heras B, Hortelano S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm Allergy Drug Targets. 2009;8(1):28-39. [PubMed ID: 19275691]. https://doi.org/10.2174/187152809787582534.
-
17.
AkhavanKarbassi MH, Yazdi MF, Ahadian H, SadrAbad MJ. Randomized DoubleBlind PlaceboControlled Trial of Propolis for Oral Mucositis in Patients Receiving Chemotherapy for Head and Neck Cancer. Asian Pac J Cancer Prev. 2016;17(7):3611-4. [PubMed ID: 27510017].
-
18.
Watanabe MA, Amarante MK, Conti BJ, Sforcin JM. Cytotoxic constituents of propolis inducing anticancer effects: a review. J Pharm Pharmacol. 2011;63(11):1378-86. [PubMed ID: 21988419]. https://doi.org/10.1111/j.2042-7158.2011.01331.x.
-
19.
Salim EI, Abd El-Magid AD, Farara KM, Maria DS. Antitumoral and Antioxidant Potential of Egyptian Propolis Against the PC3 Prostate Cancer Cell Line. Asian Pac J Cancer Prev. 2015;16(17):7641-51. [PubMed ID: 26625775]. https://doi.org/10.7314/apjcp.2015.16.17.7641.
-
20.
Rzepecka-Stojko A, Kabala-Dzik A, Mozdzierz A, Kubina R, Wojtyczka RD, Stojko R, et al. Caffeic Acid phenethyl ester and ethanol extract of propolis induce the complementary cytotoxic effect on triple-negative breast cancer cell lines. Molecules. 2015;20(5):9242-62. [PubMed ID: 26007182]. [PubMed Central ID: PMC6272161]. https://doi.org/10.3390/molecules20059242.
-
21.
Sadeghi-Aliabadi H, Hamzeh J, Mirian M. Investigation of Astragalus honey and propolis extract's cytotoxic effect on two human cancer cell lines and their oncogen and proapoptotic gene expression profiles. Adv Biomed Res. 2015;4:42. [PubMed ID: 25789268]. [PubMed Central ID: PMC4358038]. https://doi.org/10.4103/2277-9175.151251.
-
22.
Mohammadi M, Yousefi M, Habibi Z, Dastan D. Chemical composition and antioxidant activity of the essential oil of aerial parts of Petasites albus from Iran: a good natural source of euparin. Nat Prod Res. 2012;26(4):291-7. [PubMed ID: 21416453]. https://doi.org/10.1080/14786410903374819.
-
23.
Fathollahi R, Dastan D, Lari J, Masoudi S. Chemical composition, antimicrobial and antioxidant activities of Crupina crupinastrum as a medicinal plant growing wild in west of Iran. J Rep Pharm Sci. 2018;7(2):174-82.
-
24.
Hasan Abdali M, Afshar S, Sedighi Pashaki A, Dastan D, Gholami MH, Mahmoudi R, et al. Investigating the effect of radiosensitizer for Ursolic Acid and Kamolonol Acetate on HCT-116 cell line. Bioorg Med Chem. 2020;28(1):115152. [PubMed ID: 31771799]. https://doi.org/10.1016/j.bmc.2019.115152.
-
25.
Mumivand H, Rustaii A, Jahanbin K, Dastan D. Essential Oil Composition ofPulicaria dysenterica(L.) Bernh from Iran. J Essent Oil Res. 2010;13(6):717-20. https://doi.org/10.1080/0972060x.2010.10643884.
-
26.
Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colometric methods. J Food Drug Anal. 2020;10(3). https://doi.org/10.38212/2224-6614.2748.
-
27.
Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37(2):153-61. https://doi.org/10.1046/j.1365-2621.2002.00552.x.
-
28.
Kerdar T, Rabienejad N, Alikhani Y, Moradkhani S, Dastan D. Clinical, in vitro and phytochemical, studies of Scrophularia striata mouthwash on chronic periodontitis disease. J Ethnopharmacol. 2019;239:111872. [PubMed ID: 30991136]. https://doi.org/10.1016/j.jep.2019.111872.
-
29.
Chan GC, Cheung KW, Sze DM. The immunomodulatory and anticancer properties of propolis. Clin Rev Allergy Immunol. 2013;44(3):262-73. [PubMed ID: 22707327]. https://doi.org/10.1007/s12016-012-8322-2.
-
30.
Sawicka D, Car H, Borawska MH, Niklinski J. The anticancer activity of propolis. Folia Histochem Cytobiol. 2012;50(1):25-37. [PubMed ID: 22532133]. https://doi.org/10.2478/18693.
-
31.
Bankova V, Boudourova-Krasteva G, Popov S, Sforcin JM, Funari S. Seasonal Variations in Essential Oil from Brazilian Propolis. J Essent Oil Res. 1998;10(6):693-6. https://doi.org/10.1080/10412905.1998.9701012.
-
32.
de Albuquerque IL, Alves LA, Lemos TL, Dorneles CA, de Morais MO. Constituents of the Essential Oil of Brazilian Green Propolis from Brazil. J Essent Oil Res. 2011;20(5):414-5. https://doi.org/10.1080/10412905.2008.9700044.
-
33.
Bankova V, Christov R, Popov S, Pureb O, Bocari G. Volatile Constituents of Propolis. Zeitschrift für Naturforschung C. 1994;49(1-2):6-10. https://doi.org/10.1515/znc-1994-1-202.
-
34.
Clair G, Peyron L. The study of propolis essential oil. Rivista Italiana EPPOS. 1981;63:168-70.
-
35.
Pellati F, Prencipe FP, Benvenuti S. Headspace solid-phase microextraction-gas chromatography-mass spectrometry characterization of propolis volatile compounds. J Pharm Biomed Anal. 2013;84:103-11. [PubMed ID: 23807002]. https://doi.org/10.1016/j.jpba.2013.05.045.
-
36.
Petri G, Lemberkovics E, Foldvari M. Examination of differences between propolis (bee glue) produced from different floral environments. Develop Food Sci. 1988.
-
37.
Li YJ, Xuan HZ, Shou QY, Zhan ZG, Lu X, Hu FL. Therapeutic effects of propolis essential oil on anxiety of restraint-stressed mice. Hum Exp Toxicol. 2012;31(2):157-65. [PubMed ID: 21672965]. https://doi.org/10.1177/0960327111412805.
-
38.
Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436(1):69-97. [PubMed ID: 9878691]. https://doi.org/10.1016/s1383-5742(98)00019-2.
-
39.
Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59(7):865-70. [PubMed ID: 10718345]. https://doi.org/10.1016/s0006-2952(99)00380-9.
-
40.
Murad JM, Calvi SA, Soares AM, Bankova V, Sforcin JM. Effects of propolis from Brazil and Bulgaria on fungicidal activity of macrophages against Paracoccidioides brasiliensis. J Ethnopharmacol. 2002;79(3):331-4. [PubMed ID: 11849837]. https://doi.org/10.1016/s0378-8741(01)00404-4.
-
41.
Nagaoka T, Banskota AH, Tezuka Y, Saiki I, Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem. 2002;10(10):3351-9. [PubMed ID: 12150882]. https://doi.org/10.1016/s0968-0896(02)00138-4.
-
42.
Wang D, Xiang DB, He YJ, Li ZP, Wu XH, Mou JH, et al. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of colorectal cancer cells in vitro. World J Gastroenterol. 2005;11(26):4008-12. [PubMed ID: 15996024]. [PubMed Central ID: PMC4502095]. https://doi.org/10.3748/wjg.v11.i26.4008.
-
43.
Kustiawan PM, Puthong S, Arung ET, Chanchao C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac J Trop Biomed. 2014;4(7):549-56. [PubMed ID: 25183275]. [PubMed Central ID: PMC4032829]. https://doi.org/10.12980/APJTB.4.2014APJTB-2013-0039.
-
44.
Kubina R, Kabala-Dzik A, Dziedzic A, Bielec B, Wojtyczka RD, Buldak RJ, et al. The Ethanol Extract of Polish Propolis Exhibits Anti-Proliferative and/or Pro-Apoptotic Effect on HCT 116 Colon Cancer and Me45 Malignant Melanoma Cells In Vitro Conditions. Adv Clin Exp Med. 2015;24(2):203-12. [PubMed ID: 25931350]. https://doi.org/10.17219/acem/31792.
-
45.
Drigla F, Balacescu O, Visan S, Bisboaca SE, Berindan-Neagoe I, Marghitas LA. Synergistic Effects Induced by Combined Treatments of Aqueous Extract of Propolis and Venom. Clujul Med. 2016;89(1):104-9. [PubMed ID: 27004032]. [PubMed Central ID: PMC4777451]. https://doi.org/10.15386/cjmed-527.
-
46.
Abikim G, Yimit R, Tursunay A, Aerziguli T, Mutallip A. Effect of essential oils extracted from Xingjiang propolis on cell proliferation, cell cycle progression and apoptosis in human colorectal cancer cell line HTC-116. Shijie Huaren Xiaohua Zazhi. 2011;19:1469-75.