1. Background
Periodontitis is one of the widespread diseases in the mouth that destructs the attachment tissues around the teeth. The prevalence of this disease is different regarding age, gender, socioeconomic situation, and other factors (1-3). In this disease, periodontal tissues are invaded by microorganisms. A clinical sign of periodontitis are gingival inflammation and bleeding, tooth mobility, gingival recession, halitosis, alveolar bone resorption, and ultimately tooth loss. Studies show that almost 300 types of bacteria are involved in disease etiology. Among these, capnophilic bacteria (Aggregatibacter actinomycetemcomitans, Eikinella corrodens, and Capnocytophaga) and anaerobic bacteria (Porphyromonas gingivalis, P.Intermedia, F.nucleatum, T.forsythia, and so on.) play a significant role (1-3). The main goal of periodontal treatment is to remove bacterial biofilm. This is usually achieved by mechanical debridement and systemic and local antibiotic prescription (4). However, antibiotic prescription has 2 great problems. One is maintenance of therapeutic dose of antibiotic in periodontal pocket and the other is the subtype resistance of bacteria formation and change in the normal bacterial flora (4-6). Thus, use of laser for single or combined therapy interestingly increased. Er:YAG, CO2, and Nd:YAG lasers are used in dental plaque removal, gingivectomy, soft tissue correction, gingival curettage, and gingival depigmentation (7). Low-power laser (LPL) application can be effective in periodontal treatment and its complications. Several studies show the effect of LPL in soft tissue and bone repair, inflammation decreasing, and relief of periodontal treatment complications such as pain and dental sensitivity (8, 9). Other studies stated the effect of stimulating cell proliferation, anti-inflammatory effects, and immunological benefits (10). Low- level laser associated with photosensitive material called antimicrobial photodynamic therapy, or a-PDT, is proposed to reduce bacterial contamination of pocket (11, 12). According to studies on bactericidal effects of the laser, there is a dose-dependent relationship that means increased energy density to increase the destructive power of bacteria (13, 14).
One of these new methods in antimicrobial therapy is photodynamic therapy, which uses a photo-sensitive material. This material is selectively absorbed by bacteria and cancer cells and is activated with the appropriate wavelength by light (LED or laser diode). During this process, free radicals are produced that are deadly for micro-organisms. Antibiotic resistance is increasing in recent decades that cause the orientation of research projects towards developing new anti-microbial strategies. Due to the periodontal bacteria resistance to antibiotics and insufficient concentration of drugs in GCF, more interest should be pain on photodynamic therapy in periodontics (15).
In this study, colonies of bacteria Porphyromonas gingivalis are counted before and after the laser radiation in terms of in Vitro at 5, 10, 15, and 30 seconds. Finally, the purpose of this study, according to the results, in the proposed method is non-invasive photodynamic therapy as a treatment for periodontitis.
2. Methods
This research is jointly done between the school of dental medicine and Imam Khomeini hospital in Ahvaz. In this study, the bacterium Porphyromonas gingivalis cultured in 20 plates were used as samples.
After the preparation of the culture media (Luria broth) containing water, sugar, salt, and yeast extract, 3 mL of these were placed in a test tube and sterilized by autoclave. Porphyromonas gingivalis bacteria, in the log phase, was picked up using a sterile pipette tip, thrown into the test tube, and rotated; then, by using a sterile aluminum door, the test tube was closed in a way where there was only a little entry for air into the tube. Finally, the test tube was put in an incubator at a temperature of 37°C for 18 hours.
After completion of the period of reproduction and incubation to ensure the success of proliferation, test tubes that were characterized by an aura of cloud or fog were checked out. Then, by using cotton swabs, samples were placed on 20 plates. A total of 15 agar plates were chosen for the test group and 5 agar plates for control group, randomly.
Each plate was observed under a light microscope and the colonies were counted by the most probable number (MPN) method. Then, the photosensitizing agent (Methylene Blue 1%) was added to the test group plates. Diode laser (UK-QuickLase-DentaLase4w 810 nm), 810 nm wavelength, and 0.9 W/cm2 power density were applied. The laser beam in the 4th stage of the test samples were irradiated as follows:
In the 1st stage, a laser beam was irradiated for 5 seconds to test groups and immediately the number of colonies of bacteria P.gingivalis (Pg) was recorded under light microscope × 10.
In the 2nd stage, the laser beam was applied for more than 5 seconds (10 seconds in total) to the experimental groups and the numbers of colonies of bacteria were recorded.
In the 3rd stage, the laser beam was applied for more than 5 seconds (15 seconds in total) to the experimental groups and the number of colonies of bacteria was recorded.
In the 4th stage, the laser beam was applied for more than 15 seconds (30 seconds in total) to the experimental groups and the number of colonies of bacteria was recorded.
Finally, data were analyzed by SPSS version 21 and continually analyzed data were reported as mean and standard deviation (Mean ± SD) as well as discrete data as the frequency (number / percentage). To compare before and after treatment, Paired t-test was used. For quantitative comparison of groups, (K2) square were used. For the relationship between variables, regression and Pearson tests were used. The P value less than 0.05 (P < 0.5) was considered as significant.
3. Results
In this study, 20 samples were examined, 15 samples of which were the experimental group and 5 samples as the control group.
Diode laser was applied 5, 10, 15 and 30 seconds in the first, second, third and fourth times, respectively. Immediately after irradiation the number of colonies of bacteria P.gingivalis (Pg) was recorded under light microscope × 10.
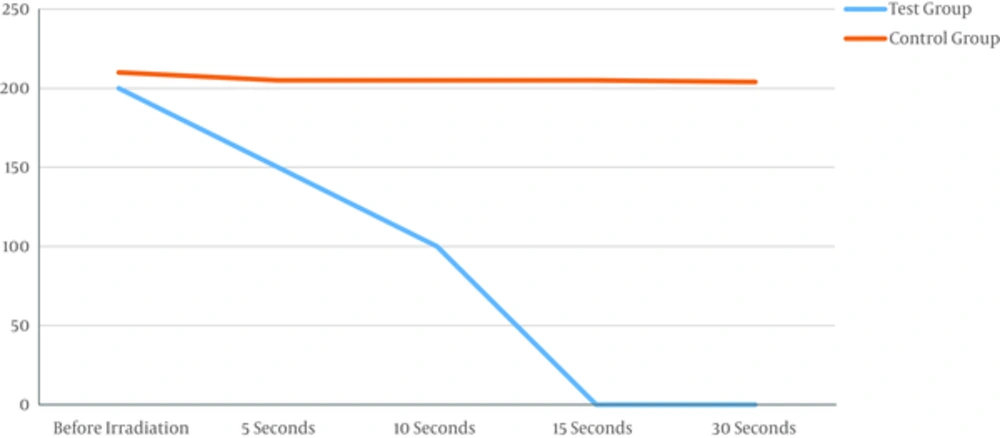
In every 15 samples, laser group responded to treatment and all the bacteria Pg died after 15 seconds of irradiation and the medium was sterilized. The difference was significant before and after laser irradiation (Table 1 and Figure 1). As the Figure 1 shows, the bacterial colonies after 5 and 10 seconds are reduced, the laser beam, but irradiation for 15 seconds completely destroys the colony and can be sterilized medium.
| Test Group (Pg Colony Count) | Control Group (Pg Colony Count) | P Value | |
|---|---|---|---|
| Before irradiation | 200 | 210 | 0.89 |
| 5 seconds | 150 | 205 | 0.04 |
| 10 seconds | 100 | 205 | 0.01 |
| 15 seconds | 0 | 205 | 0.001 |
| 30 seconds | 0 | 204 | 0.001 |
The laser is not reflected in any five plates of the control group. Checking samples showed that colonies of bacteria Pg medium remain almost unchanged. The difference was not significant (P < 0.5).
4. Discussion
As one of the most common and important disease of dental attachment tissues, Periodontitis is mainly due to the performance of anaerobic microorganisms. High prevalence of the disease can lead to tooth loss and the subsequent physical problems. According to microbial disease, the mechanical and chemical (pharmaceutical) methods, so far, has been used as main treatment options. Due to the microbial diversity, multiple medications should be used to treat. Systemic administration of most of the existing drugs has side effects. In addition, their concentration in the pathology of gum (periodontal pocket) is small (16, 17). In recent years, the use of topical medications, that although have no systemic side effects, but drug resistance and simultaneous use of several drugs still remains a problem. The use of lasers in the treatment of periodontitis has been associated with much success. The laser has good quality and high power to destroy microorganisms, significant detoxification, and bactericidal effects. Totally, lasers with 2 Photo thermal and photochemical (photodynamic therapy) effects can kill bacteria. In the photothermal method, high-energy laser damage bacteria; while in photochemical method, the bacteria will not be disappearing due to temperature rise; in addition, after laser radiation to the photo-sensitive agent (photosensitizer) attached to the bacteria, it is activated and produces toxins that kills the bacteria. Photodynamic therapy (PDT) is a procedure, which consists of exciting the photosensitizing agent to a higher energy state in the presence of oxygen by means of laser light energy (18, 19). Cytotoxic products such as singlet oxygen molecules with highly reactive energy are generated in this process, which may have a bactericidal effect on periodontal microorganisms (18). The radius action of cytotoxic products cannot usually exceed more than 0.02 μm from the radiation center. This characteristic may make PDT an appropriate noninvasive and localized treatment procedure (20). In addition, since its mechanism depends on chemically reactive molecules such as singlet oxygen and hydroxyl radicals, developing resistance to this treatment seems to be improbable (21). However, numerous studies carried out in the field of PDT, by applying diode laser light and variant photosensitizers such as malachite green (22), toluidine blue (23), phenothiazine chloride (24) and methylene blue (25), have reported conflicting clinical and microbial findings when comparing the effects of PDT adjunct to SRP with SRP alone.
The first study on the laser effect on periodontitis-causing bacteria in the laboratory is the study of Kato et al., in 1998 (17). The aim of this in vitro study was an investigation of the effect of CO2 laser in reducing Streptococcus sanguis and Porphyromonas gingivalis bacteria on titanium discs. The results of this study of CO2 laser at 268 J/cm2 and 245 J/cm2 were able to completely eliminate bacteria P. gingivalis and S.sanguis.
In this study, 20 samples were analyzed. In the laser group, samples responded to treatment and by irradiation every 15 samples for 15 seconds, the bacteria Pg died and the medium was sterilized. The difference before and after irradiation in the experimental group was significant. In the control group, which was not irradiated by laser, the samples showed that bacteria Pg colonies in culture remained almost unchanged and the difference was not significant.
The results are similar to an in vitro laboratory study that has been done before. In 2006 in an in vitro study on the effect of Nd: YAG laser on the surface of sandblast titanium, Giannini et al. investigated bacteria Escherichia coli and Aggregatibacter actinomycetemcomitans impregnated with Nd:YAG laser. They concluded that the pulsed laser Nd: YAG low pulse energy and repetition rate with any degree of clinically significant decrease can be obtained in the number of bacteria (26).
Haas et al., also studied the diode laser radiation (905 nm) with toluidine blue and showed a significant decrease of bacteria (Prevotella intermedia, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans) (27).
In another study in 2002, Kreisler et al., showed that the laser Er: YAG (Erbium: yttrium, aluminum, garnet) can eliminate more than 99% of bacteria (28). The results of the study were similar with a study by Miller, therefore, the laser Er, Cr: YSGG, with the same specifications, were used (29).
4.1. Conclusion
This study showed that low photodynamic therapy in vitro, with 10 seconds of exposure, decreased the amount of bacteria Porphyromonas gingivalis and radiation more than 15 seconds, causes the complete disappearance of the bacteria (1).