Abstract
Background:
Oral lichen planus (OLP) is a chronic inflammatory disease with muco - cutaneous involvement and unknown etiology. Endothelin1 (ET - 1) is a potent vasoconstrictor peptide, which is associated with some inflammatory diseases. The present study aimed at measuring and comparing the salivary level of ET - 1 in patients with OLP.Methods:
The current case - control study included 20 Iranian patients diagnosed with OLP as the case and 20 age - sex - matched healthy volunteers as the control groups. All the participants signed the informed consent form before recruitment. The ET - 1 level in whole unstimulated saliva (WUS) was determined by the enzyme - linked immunosorbent assay (ELISA) technique. Statistical analysis was conducted using ANOVA and t test.Results:
The mean salivary ET - 1 level in patients with OLP showed no significant difference compared with that of the control group (24.67 ± 12.07 pg/mL vs. 26.83 ± 7.73 pg/mL; P > 0.05). There were no significant difference in the salivary ET - 1 level between the reticular and erythematous - erosive groups (23.31 ± 9.12 pg/mL vs. 25.78 ± 13.99 pg/mL; P > 0.05).Conclusions:
There were no significant differences in terms of the salivary ET - 1 level between the OLP and control groups.Keywords
1. Background
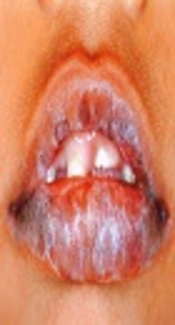
Oral lichen planus (OLP) is a chronic inflammatory disease that affects oral mucous membrane and sometimes is associated with lesions of the skin. Most of the times it represents bilateral wide striations, papules or plaques with erythema, erosions and blisters on the buccal mucosa, tongue, and gingiva (1).The estimated prevalence of OLP varies 0.5% to 2.2% in the population. It is more frequently observed in females within the age range of 30 to 60 years (2). The exact etiology of OLP is unknown, but recent data suggest that OLP is diagnosed as a cell - mediated immune disease, which damages the basal keratinocytes in oral mucosa recognized as an antigenically foreign or altered (3). Endothelin is a family with three small peptides: endothelin 1 (ET - 1), ET - 2, and ET - 3 (4). The ET - A and ET - B are cell surface receptors that mediate endothelin’s effects. Both receptors belong to the large family of G - protein - coupled receptor, and have seven transmembrane - spanning domains (5-8). It is noteworthy that most ET - 1 effects exert via interaction with ET - A receptor (9). It is recently found that ET - 1 belonging to the endothelin family of potent vasoconstrictors involves in vascular biology and mediates various pathological conditions including inflammation, wound healing, and carcinogenesis (10, 11). The role of ET - 1 is to control inflammatory responses by promoting the adhesion and migration of neutrophils and stimulating the production of pro - inflammatory cytokines (12). The analysis of salivary level of ET - 1 by the enzyme - linked immunosorbent assay (ELISA) is an easy and non - invasive method, which is recommended to detect and evaluate many diseases, and demonstrates a valuable tool for the study of oral diseases (13). According to studies, ET - 1 is presented as a potential salivary biomarker for the detection of oral squamous cell carcinoma (OSCC) (4, 13). It is well demonstrated that ET - 1 promotes growth and progression in a variety of tumors and on the other hand, it is almost accepted that patients with OLP are at high risk for OSCC (14, 15). Some studies evaluated ET - 1 level in patients with OLP; however, results of such studies are controversial (16, 17). The current pilot study aimed at investigating the salivary ET - 1 levels in patients with erythematous - erosive OLP and comparing intragroup and intergroup results with the control group.
2. Methods
The current case - control study was conducted on 20 patients with OLP (9 reticular and 11 erythematous - erosive forms). The study was designed and performed in the Oral Medicine Department of Jundishapur University of Medical Sciences, Ahvaz, Iran from 2014 to 2015. The patients were selected from the clients of School of Dental Medicine. OLP was diagnosed based on the standard clinical criteria, and confirmed by incisional biopsy. The study also included 20 age- and sex - matched healthy individuals as the control group. Patients with autoimmune diseases, immune deficiency, diabetes, cardiovascular disorder, allergic diseases, pregnant females, breastfeeding mothers, smokers, drug abusers, antibiotics or anti-inflammatory drugs consumers were exclude from the study. The study objectives and process were explained to volunteers and after signing the informed consent form, the whole saliva was collected from 10:00 AM to 01:00 PM based on a previously published protocol and matched saliva collection time (13). Briefly, participants were asked to refrain from eating, drinking, or oral hygiene procedures on the day of saliva collection. A water mouth rinse was administered prior to saliva sample collection. Five minutes after the oral rinse, the participant was asked to sit upright and spit into a Falcon tube placed on ice. Approximately, 5 mL of saliva were collected within 5 minutes. Saliva samples were processed immediately after collection according to a previously published method (16). Briefly, the saliva samples were centrifuged at 2600 g for 15 minutes at 4°C. All samples were stored at - 80°C in refrigerator until use. The salivary ET - 1 levels were measured by ELISA kit (Shanghai Crystal day Biotech Co., Shanghai, China) according to the manufacturer’s instructions. The optical density (OD) at 450 nm wavelength for each sample was recorded by microplate reader (FLU Ostar OPTIMA, BMG LABTECH, Germany). The ET - 1 concentration in each saliva sample was calculated based on the standard curve. The results were analyzed with SPSS version 20.0. The levels of ET - 1 were compared between the subjects of the groups and analyzed using t test.
3. Results
The concentration of ET - 1 in saliva samples obtained from patients with OLP (N = 20) were compared with that of the control group (N = 20). The age range of the participants was 22 to 62 years (42.44 ± 12.07 years). The age and sex distribution of the patients and 20 controls is shown in Table 1. There were no significant differences between the mean age and sex distribution of the study groups (P > 0.05). The mean salivary ET - 1 concentrations for the study groups are shown in Table 2.
Age and Gender Distribution of Patients and Control Group
| Groups | Demographic | |||
|---|---|---|---|---|
| Age (Mean), Year | Gender | Total | ||
| Females | Males | |||
| Erosive OLPa | 22 - 62 (47.18) | 10 | 1 | 11 |
| Reticular OLP | 38 - 62 (52.22) | 6 | 3 | 9 |
| OLP groups | 22 - 62 (48.91) | 16 | 4 | 20 |
| Control group | 22 - 61 (42.94) | 16 | 4 | 20 |
Salivary ET - 1 Level in of Patients and Control Groups
| ET - 1a Level | ||
|---|---|---|
| Groups | Level of ET - 1 (Pg/mL) | P Value |
| Erosive groups | 25.78 | > 0.05 |
| Reticular groups | 23.31 | > 0.05 |
| Control groups | 26.83 | > 0.05 |
| Olp groups | 24.67 | > 0.05 |
The level of salivary ET - 1 in patients and controls was 24.67 and 26.83 pg/mL, respectively. The ANOVA demonstrated no significantly difference in the salivary concentration of ET - 1 between patients with OLP and controls (P > 0.05). No significant differences were observed in salivary levels of ET - 1 between the patients with reticular and erosive forms of OLP.
4. Discussion
The present experimental study was conducted using a pilot method. The study was designed to evaluate the salivary levels of ET - 1 in patients with OLP, in comparison with the control group by means of ELISA. The study results showed no significant differences between the salivary level of ET - 1 in patients with OLP and the controls. This finding was consistent with those reported by Cheng et al., who compared the levels of salivary ET - 1 in patients with OLP patients by ELISA technique (16). They concluded no significant difference in the level of salivary ET - 1 between the patients with OLP and control groups (5.1526 ± 4.1152 vs. 4.5299 ± 3.7380 pg/mL). Despite the results of the current study, they did not exclude other systemic diseases such as cardiovascular, bone and joint, gastrointestinal, diabetes mellitus, and hypothyroidism complications.
They divided patients with OLP to disease-active (4.4209 ± 3.3467 pg/mL) and disease - inactive states (5.7947 ± 4.6473 pg/mL), and compared the results with that of control group (4.5299 ± 3.7380 pg/mL) independently. They did not show a significant difference in the salivary ET - 1 levels between the case and control groups.
In order to eliminate confounding factors, all subjects with the conditions that might affect data or the analysis of data were excluded. In addition to the factors excluded from the study by Cheng et al., the current study considered some other factors as the exclusion criteria including autoimmune, immune deficiency, recurrent allergic, and any kind of metabolic diseases. The subjects who used corticosteroids or immunosuppressant agents prior to saliva collection were also excluded.
Most recently, it was demonstrated that ET - 1 is expressed in the periodontal epithelial cells of gingival tissues (18). Also, ET - 1 in inflamed gingival tissue was strongly observed in both oral and pocket epithelial cells as well as the vascular endothelial cells. It was proposed that the expression of ET - 1 mRNA in gingival tissue might be associated with an inflammatory process (19, 20). Based on clinical examinations in the current study, most of the patients had chronic moderate periodontitis (21). The mean of ET - 1 level in the current study subjects was higher than that of the Cheng study (16); the difference between the results can be attributed to the presence of periodontitis. According to the abovementioned findings, periodontitis may play a great role in increasing ET - 1 level in the current study samples (20).
Another study mentioned that several inflammatory cytokines, such as interleukin (IL) - 1, IL - 6, and IL - 8, upregulate the production of ET - 1. On the other hand, the levels of IL - 1, IL8, and tumor necrosis factor (TNF) α increased in patients with OLP (22). The study by Yousefimanesh et al., demonstrated that the level of TNF increased in patients with periodontitis (23), therefore, the presence of periodontitis in patients with OLP patients can increase ET - 1 level. In order to exclude the influence of periodontitis as an interfering factor, the results were similar in terms of periodontitis in both groups.
Furthermore, the study also analyzed ET - 1 salivary levels in reticular and erythematous - erosive groups. There were no significant difference in the salivary ET - 1 level between reticular and erythematous - erosive groups (P > 0.05). Cheng et al., demonstrated that salivary ET - 1 level in patients with OSCC before treatment was significantly higher than that of the patients with OLP. On the other hand, results did not indicate a significant difference in the salivary ET - 1 levels among the patients with OSCC. Salivary ET - 1 is a potential biomarker to detect OSCC development in patients with OLP and it is well confirmed that ET - 1 has a correlation with other factors such as tumor growth factor (TGF) - β and angiotensin II to provoke myofibroblast transdifferentiation (16).
Xu et al., evaluated the level of ET - 1 in patients with OLP and those with oral submucous fibrosis (OSF), and showed that ET - 1 levels in oral mucous of patients with OSF were much higher than those of the patients with OLP as well as the control group, and also proved that oral mucosa of patients with OLP and normal controls released a very little amount of ET - 1 (17); they also showed no significant difference between the OLP and control groups (P > 0.05). Their results were consistent with the current study findings.
The current study was focused on molecular markers and immunology of OLP and evaluated a molecular marker (24). The small size of sample may decrease sensitivity; hence, it is recommended to perform a study with a larger sample size with longer follow - up duration.
4.1. Conclusion
There was no significant difference in ET - 1 level between the patients with OLP and the control group; therefore, it is suggested to design further studies with larder sample sizes to in order to assess the biomarker.
Acknowledgements
References
-
1.
Hegab DS, Kato AM, Sweilam MA, Abd El Gaffar ES. Serum levels of tumor necrosis factor-α in patients with lichen planus. Egypt J Dermatol Venerol. 2014;34(2):102. https://doi.org/10.4103/1110-6530.150261.
-
2.
Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, Migliorati CA, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103 Suppl:S25 e1-12. [PubMed ID: 17261375]. https://doi.org/10.1016/j.tripleo.2006.11.001.
-
3.
Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49(2):89-106. [PubMed ID: 17634721].
-
4.
Hoffmann RR, Yurgel LS, Campos MM. Evaluation of salivary endothelin-1 levels in oral squamous cell carcinoma and oral leukoplakia. Regul Pept. 2011;166(1-3):55-8. [PubMed ID: 20727373]. https://doi.org/10.1016/j.regpep.2010.08.006.
-
5.
Kusserow H, Unger T. Vasoactive peptides, their receptors and drug development. Basic Clin Pharmacol Toxicol. 2004;94(1):5-12. [PubMed ID: 14725609].
-
6.
Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacol Ther. 2006;110(3):386-414. [PubMed ID: 16219361]. https://doi.org/10.1016/j.pharmthera.2005.08.012.
-
7.
Bhalla A, Haque S, Taylor I, Winslet M, Loizidou M. Endothelin receptor antagonism and cancer. Eur J Clin Invest. 2009;39 Suppl 2:74-7. [PubMed ID: 19335749]. https://doi.org/10.1111/j.1365-2362.2009.02123.x.
-
8.
Watts SW. Endothelin receptors: what's new and what do we need to know? Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R254-60. [PubMed ID: 19907001]. [PubMed Central ID: PMC2828166]. https://doi.org/10.1152/ajpregu.00584.2009.
-
9.
Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97(3 Suppl):779-84. [PubMed ID: 12548575]. https://doi.org/10.1002/cncr.11129.
-
10.
Shah R. Endothelins in health and disease. Eur J Intern Med. 2007;18(4):272-82. [PubMed ID: 17574100]. https://doi.org/10.1016/j.ejim.2007.04.002.
-
11.
Khimji AK, Rockey DC. Endothelin--biology and disease. Cell Signal. 2010;22(11):1615-25. [PubMed ID: 20466059]. https://doi.org/10.1016/j.cellsig.2010.05.002.
-
12.
Lopez Farre A, Riesco A, Espinosa G, Digiuni E, Cernadas MR, Alvarez V, et al. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation. 1993;88(3):1166-71. [PubMed ID: 8394784].
-
13.
Pickering V, Jordan RC, Schmidt BL. Elevated salivary endothelin levels in oral cancer patients--a pilot study. Oral Oncol. 2007;43(1):37-41. [PubMed ID: 16757207]. https://doi.org/10.1016/j.oraloncology.2005.12.027.
-
14.
Gandolfo S, Richiardi L, Carrozzo M, Broccoletti R, Carbone M, Pagano M, et al. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: a follow-up study in an Italian population. Oral Oncol. 2004;40(1):77-83. [PubMed ID: 14662419].
-
15.
van der Meij EH, Reibel J, Slootweg PJ, van der Wal JE, de Jong WF, van der Waal I. Interobserver and intraobserver variability in the histologic assessment of oral lichen planus. J Oral Pathol Med. 1999;28(6):274-7. [PubMed ID: 10426201].
-
16.
Cheng YS, Rees T, Jordan L, Oxford L, O'Brien J, Chen HS, et al. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncol. 2011;47(12):1122-6. [PubMed ID: 21868280]. [PubMed Central ID: PMC3225505]. https://doi.org/10.1016/j.oraloncology.2011.07.032.
-
17.
Xu C, Peng X, Liu S, Fang C. [Quantitative and immunohistochemical analysis of endothelin-1 in oral submucous fibrosis]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2000;18(6):394-6. 418. [PubMed ID: 12539469].
-
18.
Lester SR, Bain JL, Serio FG, Harrelson BD, Johnson RB. Relationship between gingival angiopoietin-1 concentrations and depth of the adjacent gingival sulcus. J Periodontol. 2009;80(9):1447-53. [PubMed ID: 19722795]. https://doi.org/10.1902/jop.2009.080643.
-
19.
Yamamoto E, Awano S, Koseki T, Ansai T, Takehara T. Expression of endothelin-1 in gingival epithelial cells. J Periodontal Res. 2003;38(4):417-21. [PubMed ID: 12828660].
-
20.
Ansai T, Yamamoto E, Awano S, Yu W, Turner AJ, Takehara T. Effects of periodontopathic bacteria on the expression of endothelin-1 in gingival epithelial cells in adult periodontitis. Clin Sci (Lond). 2002;103 Suppl 48:327S-31S. [PubMed ID: 12193115]. https://doi.org/10.1042/CS103S327S.
-
21.
Yousefimanesh H, Robati M, Malekzadeh H, Jahangirnezhad M, Ghafourian Boroujerdnia M, Azadi K. Investigation of The Association between Salivary Procalcitonin Concentration and Chronic Periodontitis. Cell J. 2015;17(3):559-63. [PubMed ID: 26464829]. [PubMed Central ID: PMC4601878].
-
22.
Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008;46(1):15-21. [PubMed ID: 17822813]. https://doi.org/10.1016/j.bjoms.2007.07.199.
-
23.
Yousefimanesh H, Maryam R, Mahmoud J, Mehri GB, Mohsen T. Evaluation of salivary tumor necrosis factor-alpha in patients with the chronic periodontitis: A case-control study. J Indian Soc Periodontol. 2013;17(6):737-40. [PubMed ID: 24554882]. [PubMed Central ID: PMC3917202]. https://doi.org/10.4103/0972-124X.124490.
-
24.
Malekzadeh H, Robati M, Yousefimanesh H, Ghafourian Boroujerdnia M, Nadripour R. Salivary Interferon Gamma and Interleukin-4 Levels in Patients Suffering from Oral Lichen Planus. Cell J. 2015;17(3):554-8. [PubMed ID: 26464828]. [PubMed Central ID: PMC4601877].