Abstract
Background:
Stress can alter behavioral parameters, oxidative stress markers, and electrocardiographic (ECG) records. Also, metal oxide nanoparticles can affect behavioral and oxidative stress parameters in animal models, but their effects on the physiological functions of the body in stress situations and ECG changes are not yet clear.Objectives:
In this study, motor activity, ECG records, and oxidant/antioxidant balance changes were investigated following administration of nanoparticles of magnesium oxide (nano-MgO) and zinc oxide (nano-ZnO) in normal and acute restraint stressed rats.Methods:
Adult male Wistar rats were divided into 16 groups, including control (saline), restraint stress (90, 180, and 360 minutes + saline), nano-MgO, and nano-ZnO (1, 5, and 10 mg/kg, intraperitoneally) groups, with or without restraint stress of 90 minutes. Motor activities were evaluated by the elevated plus maze (EPM) and open field tests. Electrocardiographic parameters were evaluated in the 6 groups. Catalase (CAT) activity and malondialdehyde (MDA) level were measured in the cerebellum + brain stem and brain hemispheres of animals.Results:
Motor activity was decreased by the stress of 90 minutes, nano-MgO 10 mg/kg, and nano-ZnO 10 mg/kg. The PR interval and ST height were decreased by the stress of 90 minutes. The QRS interval was increased by nano-MgO 5 mg/kg, and QRS amplitude, T amplitude, and ST height were decreased by nano-MgO 5 mg/kg. The QT interval and QTc were increased by nano-ZnO 5 mg/kg, and ST height was decreased by nano-ZnO 5 mg/kg. The PR interval, QRS interval, QTc, and QT interval were increased by nano-MgO 5 mg/kg in the stress of 90 minutes, and the heart rate (HR) and ST height were decreased by nano-MgO 5 mg/kg in the stress of 90 minutes. HR, QRS interval, and QTc were increased by nano-ZnO 5 mg/kg in the stress of 90 minutes, and T amplitude and ST height were decreased by nano-ZnO 5 mg/kg in the stress of 90 minutes. The MDA level was increased by nano-MgO and nano-ZnO in the brain hemisphere and cerebellum + brain stem, and CAT activity was decreased by nano-MgO and nano-ZnO in the brain hemisphere and cerebellum + brain stem. The MDA level was increased by the restraint stress of 360 minutes in the cerebellum + brain stem while it was not changed in the brain hemispheres.Conclusions:
It seems that the side effects of these nanoparticles on motor activity could be related to the imbalance of oxidant/antioxidant systems in the cerebellum + brain stem. Also, it is possible that nanoparticles increase the effects of acute stress on changes in ECG parameters.Keywords
Antioxidant Electrocardiogram Motor activity Nanoparticles Rat
1. Background
Stress as a physiological response can negatively affect various systems of the body, for instance, the central nervous system (CNS) and cardiovascular function (1). Stress can affect motor activity; induce hypoactivity and oxidative stress markers changes in some regions of the brain (2, 3). Oxidant/antioxidant imbalance can induce damage in the brain and be involved in neurodegenerative and neuropsychiatric disorders (4). Between different parts of the brain, the cerebellum plays a central role in the coordination and correction of motor behavior; also, in the reticular formation in the lower part of the brain stem, neurons are directly involved in initiating the vertebrate’s locomotion (5).
Furthermore, stress can trigger both atrial and ventricular arrhythmias, lead to electrocardiographic (ECG) parameter changes (such as the QT interval and T wave alteration), and affect heart biorhythm (6, 7). Oxidative stress is involved in the etiology of heart attacks, ischemic strokes, and peripheral arterial disease, as well as can negatively affect myocardial calcium handling, cause arrhythmias, and contribute to cardiac remodeling (8, 9).
On the other hand, magnesium and zinc ions are involved in many physiological functions and biochemical reactions, and their deficiency can cause significant disorders in CNS and cardiovascular function (10-15).
In mammalian cardiomyocytes, zinc plays an important role in excitation-contraction coupling, and there are significant associations between low serum zinc levels and heart failure (13, 14). Many cardiovascular functions (such as hypertension, angina, and cardiac arrhythmias) are affected by magnesium deficiency (15). It has been shown that intravenous infusion of magnesium could be useful in the prevention and treatment of various cardiac arrhythmias, such as supraventricular tachycardia and atrial fibrillation (2).
With the development of nanoparticles’ usage, nanoparticles of magnesium oxide (nano-MgO) and zinc oxide (nano-ZnO, as new sources of magnesium and zinc ions) have been used in animal neurobehavioral studies in recent years (16, 17). Nanoparticles have different physicochemical properties, including small size, higher surface-to-volume ratio, and catalytic/magnetic properties compared to their conventional forms; their efficacy is also different compared to conventional forms (18). Previous studies have reported that behavioral parameters, ions level, and oxidative stress markers (such as the catalase (CAT) activity and malondialdehyde (MDA) level) were affected by nano-ZnO and nano-MgO in rats (19, 20). However, their effects on motor activity and cardiovascular function in stress situations are not yet clear.
2. Objectives
According to the above information and the lack of studies about the effects of nano-MgO and nano-ZnO on the physiological functions of the body in stress situations, in the present study, the effects of both nanoparticles on motor activity, oxidative stress markers in CNS, and ECG parameters were investigated in the stressed and non-stressed male rats.
3. Methods
3.1. Animals and Treatments
In this experimental study, male Wistar rats (210 - 230 g) were provided from the Animal House, Faculty of Veterinary, Shahid Chamran University of Ahvaz, Iran. Nanoparticles of magnesium oxide and nano-ZnO suspensions (USnano., CO, USA; CAS numbers of nano-MgO = 1309-48-4 and nano-ZnO = 1314-13-2) were prepared by sonication of dry powder in 0.9% saline and injected intraperitoneally at the doses of 1, 5, and 10 mg/kg of rat body weight (19). Animals were randomly divided into control (0.9% saline), nano-MgO (1, 5, and 10 mg/kg) and nano-ZnO (1, 5, and 10 mg/kg), restrain stress of 90, 180, and 360 minutes + 0.9% saline, and restraint stress of 90 minutes + nano-MgO (1, 5, and 10 mg/kg) or + nano-ZnO (1, 5, and 10 mg/kg) groups. Nanoparticles were determined by scanning electron microscopy (SEM; Hitachi S4160., Co, Japan). The experimental protocol is shown in Table 1. The number of animals was 6 - 10 in the behavioral tests and 6 in the biochemical and ECG parameters.
| Animals Grouping and Evaluated Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Stress Induction | Intraperitoneal Injections (Immediately After Stress Induction) | EPM Test (30 Min After Injection or Stress Induction; N = 6-10) | Open Field Test (30 Min After EPM Test) (N = 6-10) | ECG Recording (30 Min After the Open Field Test; N = 6) b | Cerebellum+ Brain Stem and Brain Hemisphere Extraction (N = 6) | Catalase Activity and MDA Level |
| Saline | - | 1 mL/kg | + | + | + | + | + |
| Nano-MgO | - | 1, 5, and 10 mg/kg | + | + | + (5 mg/kg) | + | + |
| Nano-ZnO | - | 1, 5, and 10 mg/kg | + | + | + (5 mg/kg) | + | + |
| Saline | Restraint of 90, 180, and 360 min | 1 mL/kg | + | + | + (Restraint of 90 min) | + | + |
| Nano-MgO | Restraint of 90 min | 1, 5, and 10 mg/kg | + | + | + (5 mg/kg) | + | + |
| Nano-ZnO | Restraint of 90 min | 1, 5, and 10 mg/kg | + | + | + (5 mg/kg) | + | + |
3.2. Restraint Stress Induction
Acute restraint stress for 90, 180, and 360 minutes was induced by plexiglass tubes, and components were injected immediately after stress induction. To limit habituation, each rat was submitted to only 1 session of stress induction.
3.3. Elevated Plus Maze and Open Field Tests
The rat was placed into the wooden elevated plus maze (EPM) 30 minutes after components injection or stress induction and left to move freely for 5 minutes. The total number of entrances during this time in the closed arms was calculated as motor activity (16). In the open field test, the rat was placed again in the center of the arena, and motor activity was measured by the number of crossing lines during the 5 minutes. The interval time between the 2 tests was 30 minutes.
3.4. Electrocardiographic Parameter Assessment
In 6 following groups, including control (0.9% saline), nano-MgO and nano-ZnO 5 mg/kg, stress induction for 90 minutes + saline and stress induction of 90 minutes + nano-MgO 5 mg/kg or nano-ZnO 5 mg/kg, and 30 minutes after the behavioral test, animals were anesthetized. ECG parameters, including heart rate (HR; beat per minute (BPM)), RR interval (second), PR interval (second), QRS interval (second), QT interval (second), QTc (second), QRS amplitude (mV), T amplitude (mV), and ST height (mV), were recorded by Bio-Amp from lead II during 2 - 3 minutes and monitored by a Power Lab system (AD-Instruments, Australia).
3.5. Tissue Sampling and Biochemical Assessments
Animals were sacrificed according to the guidelines using chloroform. The cerebellum + brain stem, and brain hemispheres were removed. All parts were homogenized in the phosphate buffer and centrifuged. Then, extracted supernatants were used for CAT activity and MDA level measurements (18). Briefly, CAT activity was measured based on H2O and O2 generation from H2O2. The H2O2 concentration in different parts was expressed as µmole/min/mg of wet tissue (U/mg of wet tissue). Thiobarbituric acid reactive species (TBARS) formation was used to measure MDA levels in the tissue homogenate. The tissue homogenate was added to a mix of 15% trichloroacetic acid and 0.375% thiobarbituric acid dissolved in hydrochloric acid 0.25 N. A boiling water bath was used for heating the resulting mixture (5 minutes); then, it was cooled and centrifuged (12000 rpm/10 minutes). The absorbance of the supernatant was read at a wavelength of 535 nm, and the concentration of TBARS was expressed as nM/mg of wet tissue.
3.6. Statistical Analysis
Multiple comparisons were made between groups using a 1-way analysis of variance (ANOVA) and Tukey post hoc test using InStat version 3. P values less than 0.05 were considered statistically significant. All results were expressed as mean ± SEM.
4. Results
4.1. Assessment of Motor Activity
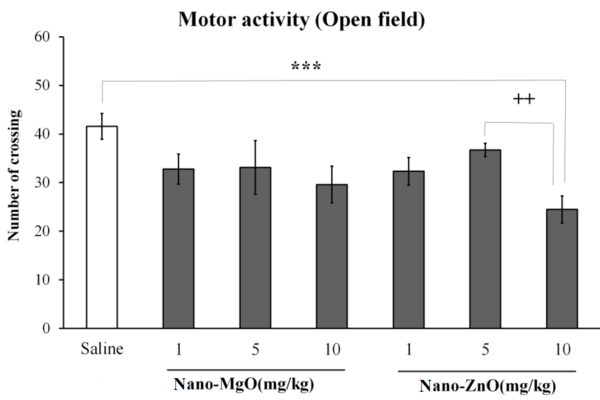
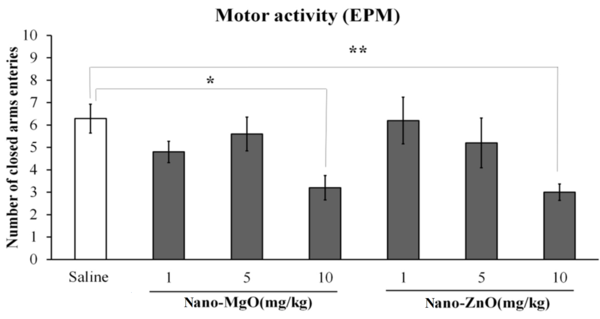
Motor activity was decreased by nano-ZnO 10 mg/kg in the open filed test (P < 0.001; Figure 1); it was also decreased by nano-MgO 10 mg/kg (P < 0.05) and nano-ZnO 10 mg/kg (P < 0.01) in the EPM test (Figure 2).
The effects of nanoparticles of magnesium oxide (nano-MgO) (1, 5, and 10 mg/kg) and zinc oxide (nano-ZnO) (1, 5, and 10 mg/kg) on motor activity in the open field test. Asterisks (***) show P values less than 0.001 compared to the control (saline) group. Pluses (++) show P values less than 0.01 compared to nano-ZnO 5 mg/kg. Data are expressed as mean ± SEM.
The effects of nanoparticles of magnesium oxide (nano-MgO) (1, 5, and 10 mg/kg) and zinc oxide (nano-ZnO) (1, 5, and 10 mg/kg) on motor activity in the elevated plus maze (EPM) test. Asterisks (* and **) show P values less than 0.05 and 0.01 compared to the control (saline) group. Data are expressed as mean ± SEM.
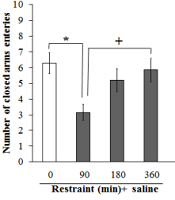
Motor activity was decreased by the stress of 90 minutes (P < 0.05) in the open field and EPM tests (Figures 3 and 4); thus, the stress of 90 minutes was selected for the following experiments.
The effects of different times of restraint, restraint (90 minutes) + nanoparticles of magnesium oxide (nano-MgO) (1, 5, and 10 mg/kg), and restraint (90 minutes) + nanoparticles of zinc oxide (nano-ZnO) (1, 5, and 10 mg/kg) on motor activity in the open field test. The asterisk (*) shows a P value less than 0.05 compared to the control (saline) group. Pluses (+ and ++) show P values less than 0.05 and 0.01 compared to the restraint (90 minutes) + nano-ZnO 1 mg/kg. Data are expressed as mean ± SEM.
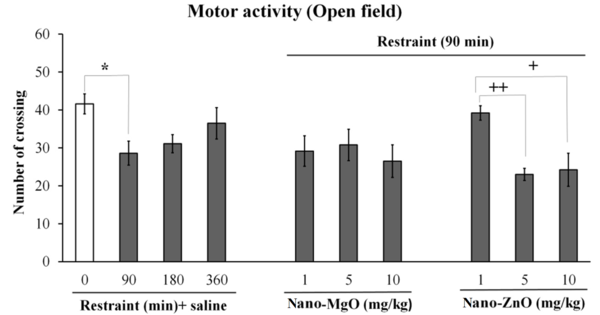
The effects of different times of restraint, restraint (90 minutes) + nanoparticles of magnesium oxide (nano-MgO) (1, 5, and 10 mg/kg) and restraint (90 minutes) + nanoparticles of zinc oxide (nano-ZnO) (1, 5, and 10 mg/kg) on motor activity in the elevated plus maze (EPM) test. The asterisk (*) shows a P value less than 0.05 compared to the control (saline) group. The plus (+) shows a P value less than 0.05 compared to the restraint (90 minutes) + saline. Data are expressed as mean ± SEM.
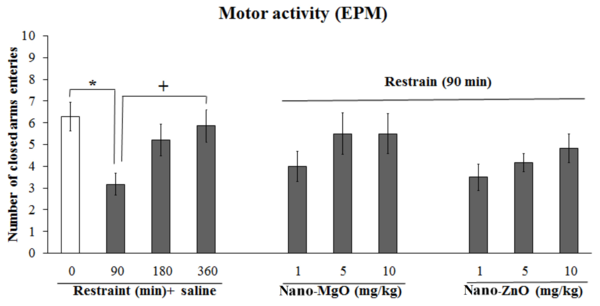
Motor activity was decreased by the stress of 90 minutes + nano-ZnO 5 and 10 mg/kg (P < 0.01 and P < 0.05, respectively) compared to the stress of 90 minutes + nano-ZnO 1 mg/kg (Figure 3).
4.2. Assessment of Electrocardiographic Parameters
Since the motor activity was affected by the stress of 90 minutes and both nanoparticles at a dose of 5 mg/kg, it could not be changed in the non-stressed rats; accordingly, they were selected to evaluate ECG parameters.
Data of ECG recording are shown in Table 2. Heart rate or RR interval were not changed by both nanoparticles or stress induction, while HR was increased by nano-ZnO 5 mg/kg (P < 0.05), and the RR interval was decreased by nano-MgO 5 mg/kg (P < 0.01) in the stressed rats. PR interval time was decreased by the stress (P < 0.05; Table 2).
| Groups | HR (BPM) | RR (s) | PR Interval (s) | QRS Interval (s) | QTc (s) | QT Interval (s) | QRS Amplitude (mV) | T Amplitude (mV) | ST Height (mV) |
|---|---|---|---|---|---|---|---|---|---|
| Control (saline) | 209.6 ± 12.458 | 0.294424 ± 0.01833 | 0.043818 ± 0.002866 | 0.014007 ± 0.001162 | 0.071583 ± 0.006889 | 0.039257 ± 0.002993 | 0.841073 ± 0.03025 | 0.2245 ± 0.007204 | 0.10941 ± 0.003457 |
| Nano-ZnO 5 mg/kg | 210.475 ± 11.112 | 0.290975 ± 0.01496 | 0.045757 ± 0.002081 | 0.01545 ± 0.000994 | 0.11494 ± 0.01437 b | 0.060297 ± 0.007888 b | 0.793125 ± 0.05583 | 0.233367 ± 0.01383 | 0.04877 ± 0.002299 c |
| Nano-MgO 5 mg/kg | 224.3 ± 6.177 | 0.28 ± 0.006223 | 0.045372 ± 0.00075 | 0.021013 ± 0.000697 c | 0.08038 ± 0.000532 | 0.042542 ± 0.000748 | 0.695433 ± 0.03014 d | 0.040995 ± 0.0309 c | -0.08236 ± 0.02 c |
| Restraint (90 min) e saline | 203.175 ± 3.53 | 0.296024 ± 0.005079 | 0.032095 ± 0.003422 b | 0.015173 ± 0.000286 | 0.080317 ± 0.003367 | 0.043743 ± 0.002157 | 0.787068 ± 0.04601 | 0.220067 ± 0.00422 | 0.07476 ± 0.004544 c |
| Restraint (90 min) e nano-ZnO 5 mg/kg | 239.6 ± 2.584 e | 0.257868 ± 0.02141 | 0.03349 ± 0.003772 | 0.025878 ± 0.001787 f | 0.09236 ± 0.001897 e | 0.047233 ± 0.001825 | 0.773935 ± 0.05021 | 0.109673 ± 0.01558 f | -0.05274 ± 0.00628 f |
| Restraint (90 min) e nano-MgO 5 mg/kg | 178.92 ± 4.674 g | 0.3371 ± 0.00904 g | 0.037953 ± 0.002514 | 0.016903 ± 0.00012 f | 0.1289 ± 0.000491 f | 0.072172 ± 0.000275 f | 0.7252 ± 0.01633 | 0.220967 ± 0.01126 | -0.01997 ± 0.004753 f |
QRS interval time was increased by nano-MgO 5 mg/kg (P < 0.01). In the stressed groups, QRS interval time was not changed by the stress alone, while it was decreased by nano-MgO 5 mg/kg (P < 0.001) and increased by nano-ZnO 5 mg/kg (P < 0.001; Table 2).
QTc time was increased by nano-ZnO 5 mg/kg (P < 0.05). In the stressed rats, QTc time was also increased by nano-ZnO 5 mg/kg (P < 0.05) and nano-MgO 5 mg/kg (P < 0.001; Table 2).
QT interval time was increased by nano-ZnO 5 mg/kg (P < 0.05), and in the stressed rats, it was increased by nano-MgO 5 mg/kg (P < 0.001; Table 2).
In the stressed rats, QRS amplitude was decreased by nano-MgO 5 mg/kg (P < 0.01; Table 2).
T amplitude was increased by nano-MgO 5 mg/kg (P < 0.001), while in the stressed rats, it was decreased by nano-ZnO 5 mg/kg (P < 0.001; Table 2).
ST height was decreased by nano-ZnO 5 mg/kg (P < 0.001), nano-MgO 5 mg/kg (P < 0.001) and stress alone (P < 0.001); it was also decreased by both nanoparticles in the stressed rats (P < 0.001; Table 2).
4.3. Catalase Activity and Malondialdehyde Level
Data on CAT activity and MDA levels are shown in Table 3. In the cerebellum + brain stem, CAT activity was decreased by nano-MgO 5 and 10 mg/kg (P < 0.01), while it was not changed by all doses of nano-ZnO; also, in the stressed rats, it was not changed by the stress induction alone or by both nanoparticles (Table 3). Furthermore, in the cerebellum + brain stem, the MDA level was increased by nano-MgO 5 mg/kg (P < 0.05), nano-ZnO 1, 5, and 10 mg/kg (P < 0.05 and P <0.01), and stress of 360 minutes (P < 0.05; Table 3).
Catalase Activity (U/mg of Wet Tissue) and Malondialdehyde Level (nM/mg of Wet Tissue) Were Measured in the Cerebellum + Brain Stem and Brain Hemispheres of All Animals a
| Groups | CAT Activity (U/mg of Wet Tissue) | MDA (nM/mg of Wet Tissue) | ||
|---|---|---|---|---|
| Cerebellum + Brain Stem | Brain Hemispheres | Cerebellum + Brain Stem | Brain Hemispheres | |
| Control (saline) | 32.24 ± 2.22 | 26.22 ± 0.77 | 2.39 ± 0.39 | 2.91 ± 0.40 |
| Nano-MgO 1 mg/kg | 31.38 ± 1.67 | 26.87 ± 1.50 | 3.36 ± 0.26 | 4.95 ± 0.39 b |
| Nano-MgO 5 mg/kg | 21.33 ± 2.47 b, c | 21.56 ± 1.16 d, e | 3.63 ± 0.21 d | 2.45 ± 0.19 f |
| Nano- MgO 10 mg/kg | 22.23 ± 0.50 b, e | 24.82 ± 0.83 | 3.54 ± 0.26 | 3.92 ± 0.41 e |
| Nano-ZnO 1 mg/kg | 31.79 ± 1.4 | 29.25 ± 3.77 | 4.32 ± 0.19 d | 3.1 ± 0.35 |
| Nano-ZnO 5 mg/kg | 33.34 ± 1.0 | 25.68 ± 1.05 | 4.67 ± 0.17 d | 4.1 ± 0.46 |
| Nano- ZnO 10 mg/kg | 31.94 ± 0.25 | 23.19 ± 0.66 | 4.81 ± 0.77 b | 4.9 ± 0.33 b, f |
| Restraint (90 min) + saline | 31.57 ± 2.21 | 23.56 ± 0.82 | 3.07 ± 0.18 | 3.2 ± 0.17 |
| Restraint (180 min) + saline | 30.53 ± 1.49 | 23.10 ± 1.14 | 2.46 ± 0.20 | 2.42 ± 0.54 |
| Restraint (360 min) + saline | 32.54 ± 2.33 | 23.89 ± 0.70 | 3.66 ± 0.39 d | 2.89 ± 0.38 |
| Restraint (90 min) + nano-MgO 1 mg/kg | 30.40 ± 0.61 | 21.78 ± 1.51 | 3.22 ± .17 | 3.35 ± 0.46 |
| Restraint (90 min) + nano-MgO 5 mg/kg | 26.1 ± 1.25 | 22.20 ± 0.73 | 3.05 ± .60 | 2.73 ± 0.16 |
| Restraint (90 min) + nano-MgO 10 mg/kg | 26.6 ± 1.82 | 19.32 ± 1.69 | 3.59 ± .26 | 2.47 ± 0.45 |
| Restraint (90 min) + nano-ZnO 1 mg/kg | 27.01 ± 0.68 | 20.96 ± 0.81 | 3.02 ± 0.28 | 3.58 ± 0.23 |
| Restraint (90 min) + nano-ZnO 5 mg/kg | 27.86 ± 1.08 | 24.46 ± 1.04 | 2.55 ± 0.36 | 3.68 ± 0.25 |
| Restraint (90 min) + nano-ZnO 10 mg/kg | 27.92 ± 0.73 | 24.07 ± 1.41 | 3.78 ± .036 | 2.9 ± .031 |
In the brain hemispheres, CAT activity was decreased by nano-MgO 5 mg/kg (P < 0.01), while the MDA level was increased by nano-MgO 1 mg/kg and nano-ZnO 10 mg/kg (P < 0.01; Table 3).
5. Discussion
In the current study, motor activity decreased, and some ECG parameters and oxidative stress markers were changed by the stress. As described before and in line with our results, stress can affect motor activity; induce hypoactivity and oxidative stress markers changes in some regions of the brain (2, 3). Also, acute mental stress can increase HR and decrease the PR interval, QT interval, and prolonged QTc interval in humans (21).
The results indicated that in non-stressed animals, motor activity was decreased by the highest dose of nano-MgO and nano-ZnO, and nano-ZnO was more effective than nano-MgO in reducing motor activity. It was shown that nano-MgO had no significant effect on motor activity in animals, while nano-ZnO could decrease it in high doses (16, 19, 22). The cerebellum and brain stem are 2 important areas involved in motor activity (5). Accordingly, the possible mechanism of nanoparticles’ effects on motor activity was evaluated to measure the oxidant/antioxidant markers in the cerebellum + brain stem and brain hemispheres.
The results indicated that CAT activity was decreased by nano-MgO, while the MDA level was increased by nano-ZnO in a dose-dependent manner. Previously, we have shown that serum ions’ levels and hippocampus oxidative stress markers could be altered following nano-ZnO and nano-MgO injection in stressed and non-stressed rats (19). It has been indicated that in CNS, nanoparticles could increase oxidative stress agents (23). In CNS, zinc ions work as an antioxidant, while nano-ZnO can elicit oxidative stress and increase the MDA level in old mice and rats’ brains (24, 25). Also, magnesium deficiency can indirectly increase the oxidative damage of biomolecules by inducing a stress response, such as a significant decrease in CAT activity (26). In the present study, oxidative stress markers were changed by both nanoparticles, especially in the cerebellum + brain stem area; these changes show the importance of this region regarding nanoparticle effects on oxidant/antioxidant changes and their relation to the motor activity.
In the other part of this study, ECG parameters were significantly changed by nano-MgO and nano-ZnO, and stress induction could promote these changes. There are not many studies on nano-MgO and nano-ZnO effects on ECG parameters. Najafzadeh Varzi et al. indicated that some ECG parameters were changed by the nano-MgO injection (27). Kesmati et al. showed that ECG parameters were changed in ovariectomized female rats following nano-ZnO injection with or without stress induction (28). Korkmaz-Icoz et al. reported that in type 2 diabetic rats, oral treatment with a zinc complex of acetylsalicylic acid could restore prolonged QT intervals (29). Treatment with this kind of zinc complex in rats following ischemic myocardial injury induction could also restore elevated ST segment and prolonged QT interval (30). Zinc ions can suppress cardiomyocyte systolic function and enhance relaxation function by changing intracellular calcium levels (31). It has been reported that in torsades de pointes (TdP) patients (a life-threatening ventricular tachycardia occurring in long QT-syndrome patients), proton pump inhibitors (PPIs) induced hypomagnesemia and increased the mean of the QT-prolonging risk factor (32).
Since ions levels in the rat’s body could be affected by nano-MgO and nano-ZnO (19, 20), this change is probably one of the most important reasons for the observed ECG parameter changes. Furthermore, nanoparticles’ effects on ECG parameters could be due to their effects on cardiovascular system structure; possible mechanisms involved in these effects can be evaluated in future studies.
5.1. Conclusions
According to the present experiment, the effects of both nanoparticles on motor activity could be due to imbalanced oxidant/antioxidant marker levels in different parts of the brain, especially in the cerebellum + brain stem. Also, it seems that acute injection of nano-MgO and nano-ZnO could affect ECG parameters, and stress could influence both nanoparticles’ effects.
Acknowledgements
References
-
1.
Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: A review. EXCLI J. 2017;16:1057-72. [PubMed ID: 28900385]. [PubMed Central ID: PMC5579396]. https://doi.org/10.17179/excli2017-480.
-
2.
Padovan CM, Guimaraes FS. Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med Biol Res. 2000;33(1):79-83. [PubMed ID: 10625878]. https://doi.org/10.1590/s0100-879x2000000100011.
-
3.
Bhagya V, Christofer T, Shankaranarayana Rao BS. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian J Pharmacol. 2016;48(6):687-93. [PubMed ID: 28066108]. [PubMed Central ID: PMC5155471]. https://doi.org/10.4103/0253-7613.194853.
-
4.
Salim S. Oxidative Stress and the Central Nervous System. J Pharmacol Exp Ther. 2017;360(1):201-5. [PubMed ID: 27754930]. [PubMed Central ID: PMC5193071]. https://doi.org/10.1124/jpet.116.237503.
-
5.
Kiehn O, Dougherty K. Locomotion: Circuits and Physiology. In: Pfaff DW, editor. Neuroscience in the 21st Century. Berlin, Germany: Springer; 2013. p. 1209-36. https://doi.org/10.1007/978-1-4614-1997-6_42.
-
6.
Lampert R. ECG signatures of psychological stress. J Electrocardiol. 2015;48(6):1000-5. [PubMed ID: 26364755]. [PubMed Central ID: PMC4624516]. https://doi.org/10.1016/j.jelectrocard.2015.08.005.
-
7.
Varejkova E, Janisova K, Myslivecek J. Acute restraint stress modifies the heart rate biorhythm in the poststress period. Sci Rep. 2019;9(1):1794. [PubMed ID: 30742021]. [PubMed Central ID: PMC6370754]. https://doi.org/10.1038/s41598-019-38523-9.
-
8.
Goszcz K, Deakin SJ, Duthie GG, Stewart D, Leslie SJ, Megson IL. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front Cardiovasc Med. 2015;2:29. [PubMed ID: 26664900]. [PubMed Central ID: PMC4671344]. https://doi.org/10.3389/fcvm.2015.00029.
-
9.
Munzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J Am Coll Cardiol. 2017;70(2):212-29. [PubMed ID: 28683969]. [PubMed Central ID: PMC5663297]. https://doi.org/10.1016/j.jacc.2017.05.035.
-
10.
Poleszak E, Szewczyk B, Kedzierska E, Wlaz P, Pilc A, Nowak G. Antidepressant- and anxiolytic-like activity of magnesium in mice. Pharmacol Biochem Behav. 2004;78(1):7-12. [PubMed ID: 15159129]. https://doi.org/10.1016/j.pbb.2004.01.006.
-
11.
Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Brain Res Rev. 2000;34(3):137-48. [PubMed ID: 11113504]. https://doi.org/10.1016/s0165-0173(00)00044-8.
-
12.
Spasov AA, Iezhitsa IN, Kharitonova MV, Kravchenko MS. [Depression-like and anxiety-related behaviour of rats fed with magnesium-deficient diet]. Zh Vyssh Nerv Deiat Im I P Pavlova. 2008;58(4):476-85. Russian. [PubMed ID: 18825946].
-
13.
Turan B, Tuncay E. Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology. Int J Mol Sci. 2017;18(11). [PubMed ID: 29137144]. [PubMed Central ID: PMC5713363]. https://doi.org/10.3390/ijms18112395.
-
14.
Yu X, Huang L, Zhao J, Wang Z, Yao W, Wu X, et al. The Relationship between Serum Zinc Level and Heart Failure: A Meta-Analysis. Biomed Res Int. 2018;2018:2739014. [PubMed ID: 29682528]. [PubMed Central ID: PMC5845493]. https://doi.org/10.1155/2018/2739014.
-
15.
Cieślewicz A, Jankowski J, Korzeniowska K, Balcer-Dymel N, Jabłecka A. The role of magnesium in cardiac arrhythmias. J Elem. 2013;18(2):317-27. https://doi.org/10.5601/jelem.2013.18.2.11.
-
16.
Torabi M, Kesmati M, Eshagh Harooni H, Varzi HN. Effect of Intra CA1 and Intraperitoneal Administration of Opioid Receptor Modulating Agents on The Anxiolytic Properties of Nano and Conventional ZnO in Male Rats. Cell J. 2014;16(2):163-70. [PubMed ID: 24567946]. [PubMed Central ID: PMC4071981].
-
17.
Kesmati M, Zadehdarvish F, Jelodar Z, Torabi M. Vitamin C potentiate sedative effect of magnesium oxide nanoparticles on anxiety and nociception in the postpartum depression model. Nanomed J. 2017;4(1):17-24.
-
18.
Wang Z. Zinc oxide nanostructures: growth, properties and applications. J Phys: Condens Matter. 2004;16(25):R829-58. https://doi.org/10.1088/0953-8984/16/25/r01.
-
19.
Torabi M, Kesmati M, Pourreza N, Najafzadeh Varzi H, Galehdari H. Neurobehavioral and biochemical modulation following administration of MgO and ZnO nanoparticles in the presence and absence of acute stress. Life Sci. 2018;203:72-82. [PubMed ID: 29678745]. https://doi.org/10.1016/j.lfs.2018.04.023.
-
20.
Kesmati M, Tamoradi N, Rezaie A, Shahriyari A, Torabi M. Evaluating behavioral, biochemical and histopathological effects of the MgO nanoparticles administration on memory in the Alzheimer-like model of male rat. Nanomed J. 2021;8(2):140-6.
-
21.
Bhide A, Durgaprasad R, Kasala L, Velam V, Hulikal N. Electrocardiographic changes during acute mental stress. Int J Med Sci Public Health. 2016;5(5):835-8. https://doi.org/10.5455/ijmsph.2016.19082015137.
-
22.
Amara S, Slama IB, Omri K, El Ghoul J, El Mir L, Rhouma KB, et al. Effects of nanoparticle zinc oxide on emotional behavior and trace elements homeostasis in rat brain. Toxicol Ind Health. 2015;31(12):1202-9. [PubMed ID: 23744884]. https://doi.org/10.1177/0748233713491802.
-
23.
Karmakar A, Zhang Q, Zhang Y. Neurotoxicity of nanoscale materials. J Food Drug Anal. 2014;22(1):147-60. [PubMed ID: 24673911]. https://doi.org/10.1016/j.jfda.2014.01.012.
-
24.
Bhalla P, Chadha VD, Dhar R, Dhawan DK. Neuroprotective effects of zinc on antioxidant defense system in lithium treated rat brain. Indian J Exp Biol. 2007;45(11):954-8. [PubMed ID: 18072539].
-
25.
Attia H, Nounou H, Shalaby M. Zinc Oxide Nanoparticles Induced Oxidative DNA Damage, Inflammation and Apoptosis in Rat's Brain after Oral Exposure. Toxics. 2018;6(2). [PubMed ID: 29861430]. [PubMed Central ID: PMC6027438]. https://doi.org/10.3390/toxics6020029.
-
26.
Zheltova AA, Kharitonova MV, Iezhitsa IN, Spasov AA. Magnesium deficiency and oxidative stress: an update. Biomedicine (Taipei). 2016;6(4):20. [PubMed ID: 27854048]. [PubMed Central ID: PMC5112180]. https://doi.org/10.7603/s40681-016-0020-6.
-
27.
Najafzadeh Varzi H, Mosallanejad B, Fatemi Tabatabaei SR, Ghanbari Birgani P. [Evaluation of electrocardiographic changes following nano magnesium oxide and magnesium oxide administration in dog]. Iranian Veterinary Journal. 2014;10(4):72-82. Persian.
-
28.
Kesmati M, Torabi M, Pourreza N, Tayebkhah M, Asadi F. Effects of anxiolytic doses of ZnO nanoparticle on ECG parameters in restraint and non-restraint ovariectomized female rats. Nanomedicine Research Journal. 2019;4(4):253-60. https://doi.org/10.22034/NMRJ.2019.04.008.
-
29.
Korkmaz-Icoz S, Al Said S, Radovits T, Li S, Brune M, Hegedus P, et al. Oral treatment with a zinc complex of acetylsalicylic acid prevents diabetic cardiomyopathy in a rat model of type-2 diabetes: activation of the Akt pathway. Cardiovasc Diabetol. 2016;15:75. [PubMed ID: 27153943]. [PubMed Central ID: PMC4858866]. https://doi.org/10.1186/s12933-016-0383-8.
-
30.
Korkmaz S, Atmanli A, Li S, Radovits T, Hegedus P, Barnucz E, et al. Superiority of zinc complex of acetylsalicylic acid to acetylsalicylic acid in preventing postischemic myocardial dysfunction. Exp Biol Med (Maywood). 2015;240(9):1247-55. [PubMed ID: 25670850]. [PubMed Central ID: PMC4935359]. https://doi.org/10.1177/1535370215570184.
-
31.
Yi T, Vick JS, Vecchio MJ, Begin KJ, Bell SP, Delay RJ, et al. Identifying cellular mechanisms of zinc-induced relaxation in isolated cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013;305(5):H706-15. [PubMed ID: 23812383]. [PubMed Central ID: PMC3761332]. https://doi.org/10.1152/ajpheart.00025.2013.
-
32.
Lazzerini PE, Bertolozzi I, Finizola F, Acampa M, Natale M, Vanni F, et al. Proton Pump Inhibitors and Serum Magnesium Levels in Patients With Torsades de Pointes. Front Pharmacol. 2018;9:363. [PubMed ID: 29731714]. [PubMed Central ID: PMC5922007]. https://doi.org/10.3389/fphar.2018.00363.