Abstract
Background:
Cutaneous leishmaniasis is endemic in 11 Iranian provinces, and almost 80% of all cases are the rural cutaneous form caused by Leishmania major. The main important drug used to treat leishmaniasis is a five-capacity antimony compound that has hinders disease recurrence, drug resistance, complications, and long treatment duration.Objectives:
It seems necessary to search for new, natural medicine to replace the use of drugs, particularly herbal compounds with no side effects.Materials and Methods:
Different dilutions of extracts were prepared with 1 mg/mL of the extracts in 96-well plates. Cultivated 106Leishmania major promastigotes in culture flasks containing RPMI1640 medium and 10% of brain heart infusion (BHI) were counted using neobar lam and poured into each well. Following 72 hours incubation, MTT solution was added to each well, and then absorbance was documented with an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm.Results:
IC50 of Allium hirtifolium (87 μg/mL) and Ziziphus spina-christi (112 μg/mL) extracts showed higher efficiency in inhibiting the growth of promastigotes over 72 hours.Conclusions:
Allium hirtifolium and Z. spina-christi extracts showed the most efficient activity in the inhibition of promastigote growth, but this was not total. The remaining plant extracts indicated ineffective or very weak efficiency on the Leishmania promastigotes. There is a possibility of achieving better results by changing the extraction method; determining materials affecting promastigotes and extracting these materials exactly; or converting extracts to other medicinal forms, such as ointment or gel.Keywords
1. Background
Leishmania is an intracellular, unicellular parasite causing a group of tropical diseases. The disease is called leishmaniasis and is caused by a protozoan of the genus Leishmania; it is able to spread between humans and some animals, such as rodents and dogs, via the bites of several types of sandflies. Leishmaniases create four forms of clinical manifestations, as follows: cutaneous, visceral, mucocutaneous, and diffuse leishmaniasis (1). Leishmaniasis is endemic to 98 countries worldwide; currently, 12 million people are affected. Moreover, 1.5 - 2 million new cases are reported annually, among which approximately 1.5 million cases are affected by cutaneous leishmaniasis. The disease kills 20,000 to 40,000 people all over the world each year. About 90% of cases of cutaneous leishmaniasis worldwide occur in 10 countries, one of which is Iran (2).
Cutaneous leishmaniasis is endemic to Iran, and according to reports by the centers for disease control and management, the annual number of patients with various forms of leishmaniasis (cutaneous and visceral) is 25,000 cases in the country. Cutaneous leishmaniasis is endemic to 11 Iranian provinces, and Khorasan (in the east), Fars (in the southeast), and Isfahan (in the central) have the highest cases of leishmaniasis, where almost 80% of all cases represent the rural cutaneous form (3).
The disease manifestations differ based on the type of ulcers. In the wet or rural form caused by Leishmania major, an acute infection arises in a period of 3 - 6 months. Injuries occur as wet ulcers, which are mainly present on the lower limbs (1, 2, 4).
The main drug used to treat leishmaniasis is a five-capacity antimony compound that prevents the oxidation of fatty acids by parasites. Three- and five-capacity antimonies, such as glucantime, Pentostam, and allopurinol, as well as antifungal drugs like amphotericin, are used for the treatment of leishmaniasis. However, topical medications, such as mepacrine, berberine, and paromomycin, along with gentamicin, are also applied. Nevertheless, there are problems with the use of these medications, such as disease recurrence, drug resistance, complications of the drug, high cost, and long treatment duration. Therefore, it seems necessary to search for new, natural medicine compounds with no side effects to replace the drugs in use (4, 5).
The current study was planned to investigate the effect of new medicinal plant extracts with no side effects for the treatment of cutaneous leishmaniasis. Diseases have been treated with medicinal plants refers throughout human history. The use of plants in the treatment of diseases has been common in Iran for a long time, and Iranian therapists have paid special attention to this type of treatment (6).
2. Objectives
In the current study, quantitative methods were applied to examine the anti-leishmanial effect of plant extracts, such as counting using a Neubauer chamber and the MTT colorimetric method (7-9). The present study aimed to evaluate the in vitro effect of hydroalcoholic extracts of Conocarpuserectus, Ziziphus spina-christi, Eucalyptus olida, Allium hirtifolium, Descurainia sophia, and Punica granatum peel on Leishmania major promastigotes. These extracts have already indicated good anti-parasitic and antimicrobial effects in previous studies.
3. Materials and Methods
This study was carried out on Leishmania major (MRHO/IR/75/ER) prepared at the Pasteur institute of Iran.
3.1. Preparation of Plant Extracts
Plants were prepared from nature and herbal medicines, and their herbarium codes were obtained from the school of pharmacy, Ahvaz Jundishapur University of Medical Sciences. The plants collected from nature were dried in shadow, ground, soaked with 70% methanol, and shaken for 72 hours. Then, scum was separated using filter paper, and the obtained liquid was thickened using a rotary device at a temperature of less than 50°C. The obtained liquid was dried using a freeze dryer and stored in dark containers in a freezer until testing (10).
3.2. Parasite Culture
After adding phosphate-buffered saline (PBS) and brain heart infusion (BHI) to the environment of NNN and observing sufficient growth, promastigotes were cultivated in culture flasks containing RPMI1640 medium and 10% of BHI; they were then transferred into an incubator at 25 - 27°C.
3.3. Control Drug
In the present study, Meglusan (a five-capacity antimonial drug) was alternatively applied as control drug. This is imported from Belgium and used for the treatment of cutaneous leishmaniasis in Khuzestan health networks due to sanctions and the lack of glucantime drug.
3.4. The MTT Colorimetric Method
In 1983, MTT was proposed as an alternative for radioactive method; here, an enzymatic method is used as the reaction substrate of tetrazolium salt solution. An enzyme-linked immunosorbent assay (ELISA) reader measures the amount of optical density of insoluble formazan color created from tetrazolium as a result of the succinate dehydrogenase enzyme activity of parasites, and this is applied as a component of growth and viability of promastigotes against drug response (7).
3.5. Extract Dilution Preparation
One milligram of dried extract was dissolved with 50 μL of dimethyl sulfoxide (DMSO) and mixed well. Then, 950 μL of RPMI1640 medium was added to the solution and a concentration of 1 mg/ml of extract was obtained. Subsequently, 7 different dilutions (1 mg/mL, 500, 250, 125, 62.5, 31.25, and 15.62 µg/mL) of each extract were prepared in 96-well plates.
3.6. Parasite Culture Plates
Parasites cultured in several culture flasks containing RPMI1640 and BHI were mixed, and cells were centrifuged and washed using sterile PBS. Subsequently, the number of promastigotes was counted per milliliter of culture medium using a Neubauer chamber, and the obtained solution was diluted so that each well contained 106 of promastigotes. Subsequent to the extract dilution, 100 μL of parasites was added to each well and 100 μL of culture medium of RPMI1640 was added to a row without parasites as a blank. In another row, 100 μL of medium containing parasites without the drug was added, and the plate was finally put in the incubator for 72 hours. After 72 hours, MTT solution was added to the wells (the solution was made of a combination of 5 mg of MTT powder and 1 cc of sterile PBS). MTT solution was added to each well in the amount of 10% volume of the solution (11 mL), and the plate was kept in the incubator in the dark for 3 hour. Subsequently, 100 μL of DMSO was added to each well to stop the reaction. The plate was kept in a dark environment for 10 - 20 minutes. Finally, the optical density was measured at a wavelength of 570 nm using an ELISA reader (11).
4. Results
Using the following formula, the viability of promastigotes at 72 hours was calculated:
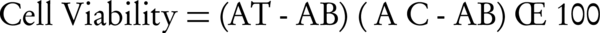
Where AT, AC, and AB are the optical absorption of samples (extracts and parasites), the optical absorption of control drug, and the optical density of the blank well, respectively (10). After calculating the viability of promastigotes using the interpolation test, the IC50 values of extracts were measured at 72 hours (Figure 1). The following values were observed for the extracts: C. erectus, 201 (μg/mL); Z. spina-christi, 112 (μg/mL); E. olida, 142 (μg/mL); A. hirtifolium, 87 (μg/mL); D. sophia, 175 (μg/mL); and P. granatum peel, 138 (μg/mL). Allium hirtifolium extract showed the most effective activity on the inhibition of growth of promastigotes.
IC50 of Extracts and Control Drug After 72 Hours

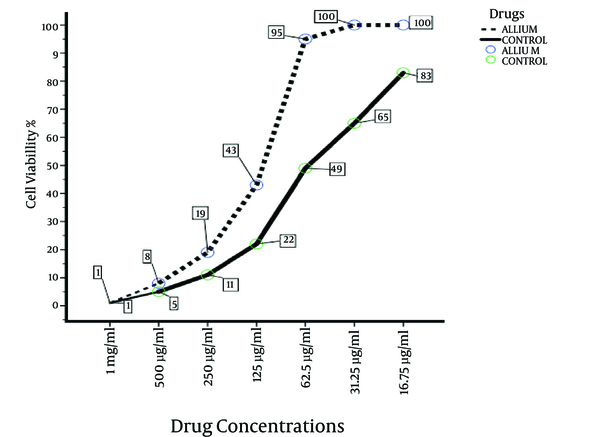
Percentage of Cell Viability of the Most Effective Extract Compared to the Control Drug After 72 Hours
4.1. Statistical Analysis
The mean optical density of three experiments of each extract was measured against the control drug using SPSS ver. 16 software. The results indicated that the mean optical density of the first three concentrations of extracts (P > 0.001) was significant compared to the control drug (P < 0.05). The effectiveness of the extracts was subsequently measured in relation to the control drug via the post hoc–Dunnett’s test. The results of the test indicated a significant difference for the first three concentrations of all extracts compared to the control drug (P > 0.001).
5. Discussion
Leishmaniasis has recently become a serious health problem in Iran. Furthermore, the long-term and expensive treatment, sanctions, lack of access to standard and efficient drugs, and the advancement of pharmacists’ success in reducing the side effects of chemical drugs have obliged researchers and even sometimes traditional healers to use natural products for the treatment of leishmaniasis in this country. Having said that, the issues above point to the need for applied and further studies on the treatment of leishmaniasis with natural and herbal elements (12). In this study, the effects of hydroalcoholic extracts of six Iranian herbs were investigated; these herbs have previously been used as antimicrobial, anti-parasitic, and antifungal remedies in traditional medicine. In addition, the current study considered accessible, affordable plants that grow in most parts of the country.
The primary studies showed that the peel of P. granatum, C. erectus leaves, and E. olida leaves have tannin, which is a phenolic compound with high molecular weight and antimicrobial efficacy. These studies also indicated anti-inflammatory and antioxidant compounds in P. granatum peel, A. hirtifolium, and D. sophia that have wound antiseptic properties. In addition, alkaloids, aldehydes, tannins, and peptides were found in the leaves of Z. spina-christi. Previous studies on plants at the same family were a significant basis for the selection of plants for the current study (13-19). The selected plants exhibits the presence of a series of anti-microbial and parasitic compounds; for example, in traditional middle eastern medicine, C. erectus is used as a skin disinfectant, relieving acne and skin wounds. These plants have also been proven to have tannin compounds with antibacterial, anticancer, and antioxidant properties (13, 14). Allium hirtifolium has been approved for antibacterial and anti-parasitic compounds, such as dialk(en)yl sulfides, allyl alcohol, and thiosulfinates, among which, allicin is the most important compound (15). Nitazoxanide in P. granatum peel is also a compound in which an anti-parasitic property has been demonstrated (16). The presence of compounds such as polyphenols and terpenoids in E. olida (17); kaempferol, quercetin, and isorhamnetin, as well as flavonoid compounds in D. sophia (18); and several types of flavonoids, alkaloids, and saponins in the leaves of Z. spina-christi with anti-parasitic and microbial effects (19) increase the potential effectiveness of these plants’ extracts on Leishmania major promastigotes.
The results of this study indicated the more than 50% growth inhibition for Leishmania promastigotes at concentrations of 1 mg/mL and 500 μL/mL for all extracts after 72 hours of incubation. The viability percentage of promastigotes against A. hirtifolium extract was very close to the control drug at 72 hours of incubation. An interesting point was that almost all extracts had the ability to inhibit the growth of more than half of parasites at a concentration of 500 μL/mL. However, these results showed that all applied crude extracts had no or little anti-leishmanial activity in vitro for the promastigote forms at concentrations of 62.5, 31.25, and 15.62 μg/mL.
Given the result of previous studies, before this research was initiated, we expected that the selected plant extracts would show better activity against Leishmania promastigotes; however, it came to our attention that this was an optimistic view. Having said this, there is a possibility of achieving better results by changing the extraction method, determining materials affecting promastigotes, and applying precise extraction methods or converting extracts to other medicinal forms such as ointment or gel. For example, in a similar study, the effect of thyme extract on L. major promastigotes was measured through the colorimetric method, and the results indicated the ineffectiveness of the extract in vitro; meanwhile, in a study conducted on Balb/c mice with L. major, hydroalcoholic thyme extract on based gel and ointment showed favorable activity (20). Considering the abovementioned cases in addition to in vitro or in vivo experiments, further examinations and tests are recommended.
5.1. Conclusion
Through this study, we concluded that plant extracts showed different growth inhibition activities against promastigotes during in vitro experiments. Inhibition of the growth rate was significantly reduced at extract concentrations of 1 mg/mL and 500 μL/mL; however, the other whole crude plant extracts showed different biological activity behaviors, probably due to the effectiveness of the compounds. Furthermore, A. hirtifolium and Z. extracts showed the most efficient activity on the inhibition growth of promastigotes. The remaining plant extracts indicated ineffective or weak efficiency on the Leishmania promastigotes.
Acknowledgements
References
-
1.
Foundations of parasitology. Parasitology Today. 1985;3(2):987.
-
2.
Simon GL, Hotez PJ, Tsuji M, Satoskar AR. Medical parasitology. Austin, Texas USA: Landes Bioscience; 2009.
-
3.
Yaghoobi-Ershadi MR, Marvi-Moghadam N, Jafari R, Akhavan AA, Solimani H, Zahrai-Ramazani AR, et al. Some Epidemiological Aspects of Cutaneous Leishmaniasis in a New Focus, Central Iran. Dermatol Res Pract. 2015;2015:286408. [PubMed ID: 26483838]. https://doi.org/10.1155/2015/286408.
-
4.
John DT, Petri WA, Markell EK, Voge M. Markell and Voge's medical parasitology. Elsevier Health Sciences. 2006.
-
5.
Akerele O. Traditional medicine, update from the World Health Organization, Geneva. Am J Chin Med. 1983;11(1-4):1-4. [PubMed ID: 6660196]. https://doi.org/10.1142/S0192415X83000021.
-
6.
Oskuee RK, Jaafari MR, Amani S, Ramezani M. Evaluation of leishmanicidal effect of Euphorbia erythadenia extract by in vitro leshmanicidal assay using promastigotes of Leishmania major. Asian Pacific J Tropic Biomed.
-
7.
Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol Int. 2005;54(2):119-22. [PubMed ID: 15866473]. https://doi.org/10.1016/j.parint.2005.01.001.
-
8.
Sengupta S, Chowdhury S, Bosedasgupta S, Wright CW, Majumder HK. Cryptolepine-Induced Cell Death of Leishmania donovani Promastigotes Is Augmented by Inhibition of Autophagy. Mol Biol Int. 2011;2011:187850. [PubMed ID: 22091398]. https://doi.org/10.4061/2011/187850.
-
9.
Eissa MM, Amer EI, El Sawy SM. Leishmania major: activity of tamoxifen against experimental cutaneous leishmaniasis. Exp Parasitol. 2011;128(4):382-90. [PubMed ID: 21620834]. https://doi.org/10.1016/j.exppara.2011.05.009.
-
10.
Khademvatan S, Adibpour N, Eskandari A, Rezaee S, Hashemitabar M, Rahim F. In silico and in vitro comparative activity of novel experimental derivatives against Leishmania major and Leishmania infantum promastigotes. Exp Parasitol. 2013;135(2):208-16. [PubMed ID: 23872452]. https://doi.org/10.1016/j.exppara.2013.07.004.
-
11.
Allahdin S, Khademvatan S, Hashemitabar M, Eskandari A. IN vitro activity of camellia sinensis extracts against L. MAJOR and L. infantum promastigotes using the colorometric mtt assay. Urmia Med J. 2014;25(10):893-900.
-
12.
Dehghani R, Kassiri H, Mehrzad N, Ghasemi N. The Prevalence, laboratory confirmation, clinical features and public health significance of cutaneous leishmaniasis in Badrood city, an old focus of Isfahan Province, Central Iran. J Coastal Life Med. 2014;2(4):319-23.
-
13.
Hameed E. Phytochemical Studies and Evaluation of Antioxidant, Anticancer and Antimicrobial Properties of Conocarpus erectus L. Growing in Taif, Saudi Arabia. European J Med Plants. 2012;10(2):2.
-
14.
Shohayeb M, Hameed EA, Bazaid S. Antimicrobial activity of tannins and extracts of different parts of Conocarpus erectus L. Int J Pharm Bio Sci. 2013;3:544-53.
-
15.
Ismail S, Jalilian FA, Talebpour AH, Zargar M, Shameli K, Sekawi Z, et al. Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium Boiss. Biomed Res Int. 2013;2013:696835. [PubMed ID: 23484141]. https://doi.org/10.1155/2013/696835.
-
16.
Al-Mathal EM, Alsalem AM. Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum. Exp Parasitol. 2012;131(3):350-7. [PubMed ID: 22580265]. https://doi.org/10.1016/j.exppara.2012.04.021.
-
17.
Elaissi A, Salah KH, Mabrouk S, Larbi KM, Chemli R, Harzallah-Skhiri F. Antibacterial activity and chemical composition of 20 Eucalyptus species' essential oils. Food Chem. 2011;129(4):1427–34.
-
18.
Lee YJ, Kim NS, Kim H, Yi JM, Oh SM, Bang OS, et al. Cytotoxic and anti-inflammatory constituents from the seeds of Descurainia sophia. Arch Pharm Res. 2013;36(5):536-41. [PubMed ID: 23435946]. https://doi.org/10.1007/s12272-013-0066-x.
-
19.
Seyyednejad SM, Motamedi H, Safary A, Maleki S. Ziziphus spina-christi, a Native Plant from Khuzestan, Iran, as a Potential Source for Discovery New Antimicrobial Agents. Asian J Plant Sci. 2009;8(2):187-90.
-
20.
Shirani-Bidabadi L, Mahmoudi M, Saberi S, Zolfaghar-Baghbaderani A, Nilforoushzadeh MA, Abdoli H, et al. The effectiveness of mix extracts of Thyme, Yarrow and Propolis on Cutaneous Leishmaniasis: a comparative study in animal model (Balb/c) [in Persian]. Tehran Univ Med J. 2009;66(11):785-90.