Abstract
Background:
Exendin-4 is a similar peptide to GLP-1 and can interfere with various receptors of GLP-1. Bisphenol A (BPA), as an endocrine disruptor chemical, is used in a wide range of plastic products.Objectives:
The aim of this study was to clarify several exendin-4 aspects in treatment of metabolic disorders.Methods:
In in vivo condition, the BPA (100 µg/kg) and exendin-4 (4 nmol/kg/d) were administered for 20 days in four different groups, and blood glucose, insulin, lipid profile, adipocyte hormones and oxidative stress markers were examined.Results:
The results indicate that co-administration of exendin-4 and BPA modified hyperglycemia and hyperlipidemia induced by BPA. Bisphenol A reduced antioxidant level, whereas exendin-4 improved these effects.Conclusions:
The findings of these studies suggest that the use of exendin-4 is useful in hyperglycemia, hyperlipidemia and metabolic disorders, induced by BPA.Keywords
Bisphenol A Exendin-4 Leptin Adiponectin Oxidative Stress Lipid Profile
1. Background
Metabolic disorder is a series of risk factors that cause health complications such as diabetes and cardiovascular disease (1). Nowadays phenolic pollutants such as Bisphenol A (BPA) are common endocrine disruptor chemicals (EDCs) that intervene with classic and non-classic receptors of estrogen. Inappropriate activation of estrogen receptors is associated with problems such as type ΙΙ diabetes and metabolic disorders (2-4).
Bisphenol A, as an EDC, is the constituent unit of polycarbonate and epoxy resins, which are used in a wide range of products such as canned food lining, dental sealants, thermal papers, plastic packs and indoor air (5-7). The direct release of BPA to the environment occurs at the processing step, through release to the air, soil and ground penetration. According to environmental protection agency of American, the release of BPA to the surrounding is more than one million pounds around the globe (8). Thus, it is not surprising that BPA is visible in foods, polluted air, soil, drinking and ground water.
Exendin-4 is a 39 amino acid peptide that has 53% sequence similarity to GLP-1 and can interfere with various receptors of GLP-1, but exendin-4 effects on metabolism is more sustained and potent than GLP-1 (9-12). Several studies have indicated that in hyper and normoglycemic conditions, exendin-4 augments insulin secretion and glucose homeostasis (13) and by affecting gastric emptying, appetite lowering and amelioration in insulin sensitivity, can be useful in treatment of metabolic disorders (14).
Induction of oxidative stress by BPA has been confirmed by various studies, so with regards to glucose reducing effect of exendin-4, which has a direct effect on oxidative damages, it is likely that exendin-4, by good impacts can heal the side effects of BPA and can be helpful in treatment of metabolic syndrome.
2. Methods
2.1. Animal
One week before beginning the experiment, 32 Naval medical research institute (NMRI) male mice (25 - 30 g body weight) aged 2.5-3 months were obtained from the animal house of Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran). Animals were accommodated in BPA free cages (22 ± 2°C, under a standard 12-hour light: dark cycle) and allowed standard ad libitum free access to food and water, instituted and divided to four groups of eight mice. The groups included the, control group (received solvent each day), the BPA group (100 µg/kg/d BPA for 20 days), the BPA + exe-4 group (100 µg/kg/d BPA for 20 days with co-administration of 4 nmol/kg/d exendin-4 in the last 10 days), and the exe-4 group (4 nmol/kg/d exendin-4 for 20 days). All protocols were compatible with standards of animal care, specified by the ethics commission (CMRC-96) of the Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran).
Based on others experiments, it was decided to examine 100 µg/kg/day of BPA (Sigma Aldrich Company, Germany) (15-17) and according to our results in dose response experiment on insulin secretion of isolated islet, at in vitro condition, choose 4 nmol/kg/day of exendin-4. The solvent of BPA was ethyl alcohol with a final concentration of 0.1% and solvent of exendin-4 was distilled water. Single dose of BPA through subcutaneous injection and exendin-4 via intra peritoneal applied per day. The treatment period choose according to other previous studies (16, 18).
2.2. Blood Collection and Biochemical Assay
24 hours after last treatment, the fasted animals anesthetized with ketamine (60 mg/kg) and xylazine (10 mg/kg) mixture at 08:00 - 09:00 am then, blood glucose level assessed by tail sampling using glucometer (Elegance, Taiwan). Blood sample collected through the cardiac puncture and centrifuged at 3000 g for 15 minutes then plasma separated and kept at -20°C. Measurements of triglyceride, total cholesterol, LDL-cholesterol (LDL-c) and HDL-cholesterol (HDL-c) concentration in plasma performed using Pars Azmoon kits and auto-analyser and VLDL-cholesterol (VLDL–c) concentration calculated by Norbert formula (VLDL = triglycerides (mg/dL)/5). Serum adiponectin level evaluated by mouse adiponectin ELISA kit (BioVendor NO: RD293023100R, Germany) with 2.3% coefficient of variation assay. The serum level of leptin assessed by mouse and rat leptin ELISA kit (BioVendor NO: RD291001200R, Germany) with 2.3% coefficient of variation assay.
2.3. Measurement of Antioxidant Activity
Pancreas tissue of treated mice examined for Catalase (CAT), glutathione peroxidase (GPX) and, superoxide dismutase (SOD) activities using microplate format detection kits (Biocore Diagnostik Ulm GmbH. Germany). The ZELL Bio Gmbh kit (cat. No: ZB-TAC-A48, V4527, Germany) used in order to assessment of TAC level in plasma and the results presented in µM/l plasma and the ZELL Bio Gmbh kit (cat. No: ZB-MDA-A48, V405, Germany) used for measurement of MDA plasma level and the results presented in µM/L plasma.
2.4. Statistical Analysis
Normal distribution and homogeneity of the variances were evaluated using Levene’s test, then one-way analysis of variance (ANOVA) followed by Tukey’s as post hoc test, also repeated measurement applied for body weight. Differences were considered statistically significant at the P value < 0.05.
3. Results
All experimental groups presented increase in body weight compared to the beginning of the study. Further analysis showed that all treatment groups revealed significant lower body weight compared to control (Table 1).
In addition, BPA by increase in blood glucose 46% (P < 0.001), induced hyperglycemia and exendin-4 compensate this effect. The serum insulin level in the BPA group decreased about 57% (P < 0.01) as compared to the control group, and co-administration of BPA and exendin-4 compensated this decrease and it was similar to the control group. Exendin-4 alone did not change serum insulin level as compared to the control group (Table 2).
Effect of Bisphenol A and Exendin-4, Alone and in Combination, on Lipid Profile, Serum Insulin and Blood Glucose (n = 7)a
| Groups | Control | BPA | BPA + exe-4 | exe-4 |
|---|---|---|---|---|
| Triglyceride, mg/dL | 50.33 ± 1.45 | 71.75 ± 6.96b | 59.5 ± 2.02c | 55.55 ± 4.29 |
| Cholesterol, mg/dL | 87.3 ± 1.2 | 104.5 ± 2.59d | 98.25 ± 8.64 | 97 ± 4.45 |
| HDL-c, mg/dL | 74.10 ± 2.30 | 62.75 ± 2.25d | 69.25 ± 4.10 | 74.04 ± 2.32 |
| LDL-c, mg/dL | 10.27 ± 0.32 | 27.4 ± 1.58b | 17.1 ± 1.42c | 11.9 ± 0.14 |
| LDL/HDL ratio | 0.14 ± 0.03 | 0.44 ± 0.09b | 0.25 ± 0.07c,d | 0.16 ± 0.05 |
| Blood glucose, mg/dL | 99.25 ± 4.58 | 145.43 ± 3.91e | 115.44 ± 3.36f | 103.28 ± 5.76 |
| Serum insulin, ng/mL | 1.42 ± 0.04 | 0.61 ± 0.08b | 1.52 ± 0.23g | 1.65 ± 0.41 |
As shown in Table 2, BPA caused an increase in triglyceride by 42% (P < 0.01), total cholesterol by 14.7% (P < 0.05), low density lipoprotein-cholesterol (LDL-c) by 166% (P < 0.01) and decrease in high density lipoprotein-cholesterol (HDL-c) by 15.3% (P < 0.05), compared with the control group. Co-administration of BPA and exendin-4 excluding LDL-c, impaired lipid profile to the normal values. In addition, exendin-4 alone induced change in the mentioned parameters.
Evaluation of oxidative parameters showed that BPA induced a significant decrease in pancreatic plasma glutathione peroxidase (GPX) (P < 0.05), total thiol concentration (P < 0.01) and plasma level of Total Anti-oxidant Capacity (TAC) (P < 0.05) and significant increase in plasma Malondialdehyde (MDA) (P < 0.01). In the current experiment, BPA did not induce any significant decrease in the superoxide dismutase (SOD) and catalase (CAT). In addition, co-administration of BPA and exendin-4 regulated the TAC, MDA and GPX as much as the control group. Exendin-4 caused a significant increase in TAC, total thiol concentration, and catalase as compared with the control group (Table 3).
Effect of Bisphenol A and Exendin-4, Alone and in Combination, on Pancreas and Plasma Antioxidant Defensea
| Group | Control | BPA | BPA + exe-4 | exe-4 |
|---|---|---|---|---|
| SOD, U/mg tissue | 0.3 ± 0.02 | 0.21 ± 0.03 | 0.26 ± 0.01 | 0.36 ± 0.05 |
| Catalase, U/mg tissue | 0.55 ± 0.06 | 0.39 ± 0.06 | 0.85 ± 0.09b,c | 0.89 ± 0.1b |
| GPX, U/mg tissue | 10.7 ± 0.74 | 7.1 ± 0.89b | 10.22 ± 1.62 | 10.38 ± 0.53 |
| Total thiol, mM/mg tissue | 87.28 ± 2.32 | 59.25 ± 11.58d | 98.4 ± 4.42e | 110.46 ± 11.1b |
| TAC, µM/l plasma | 103.05 ± 5.9 | 44.73 ± 4.8b | 81.94 ± 10.9 | 159 ± 26d |
| MDA, µM/l plasma | 2.8 ± 0.49 | 6.64 ± 1.18d | 2.41 ± 0.45e | 1. 59 ± 0.53 |
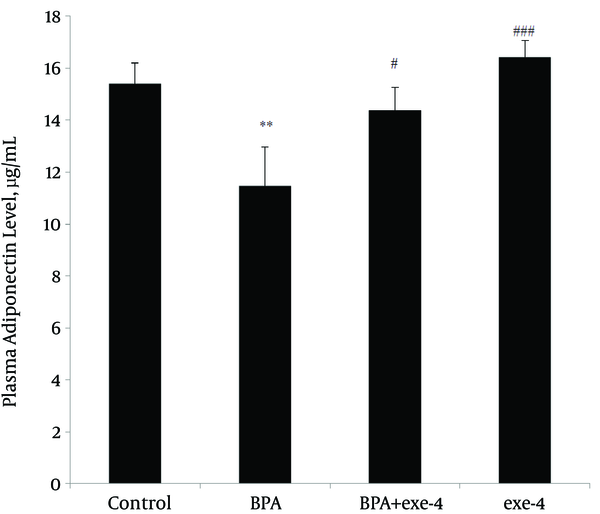
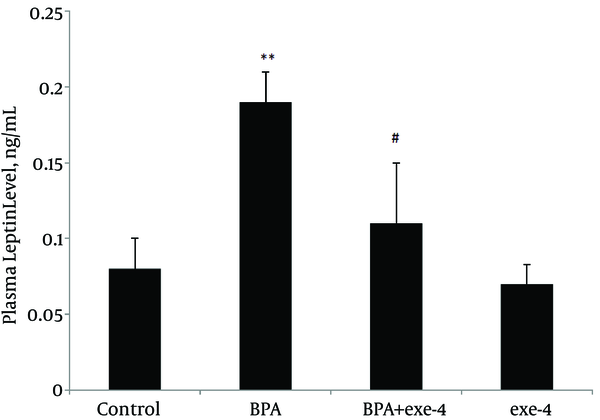
Evaluation of plasma leptin and adiponectin levels in different groups showed that BPA reduced plasma adiponectin level (P < 0.01). Furthermore, co-administration of BPA with exendin-4 and exendin-4 alone kept adiponectin level at similar values with the control group (Figure 1). Assessment of plasma leptin revealed that BPA increased this hormone (P < 0.01) and co-administration of BPA with exendin-4, and exendin-4 alone modified it to normal values (Figure 2).
Effect of BPA and Exendin-4, Alone and in Combination, on Plasma Adiponectin Level
Effect of BPA and Exendin-4, Alone and in Combination, on Plasma Leptin Level
4. Discussion
The main purpose of this study was to clarify several aspects of exendin-4 that protect subjects from metabolic disorders. The general detection of this study indicated that administration of BPA was related to diabetes and metabolic disorders. The results showed that BPA by increase in blood glucose, triglyceride, total cholesterol level, LDL/HDL ratio, plasma MDA and decrease in plasma level of insulin and HDL-c, TAC, Gpx and total thiol concentration might contribute in oxidative stress and metabolic syndrome. All the above disturbances were not seen in BPA and exendin-4 co-administration and in the exendin-4 group. Body weight increased in all experimental groups, however at the end of the procedure, the treated ones with BPA and exendin-4 had lower weight compared with the control. Some writers revealed a correlation between BPA exposure and body weight elevation (19, 20). This result may be explained by the fact that several important mechanisms may affect body weight including, glucose uptakes and homeostasis, adipocyte deposition, dose and duration of treatment, gender and species (21). In the current experiment, exendin-4 regulated blood glucose, however Liu et al. (2013) claimed that regulation of blood glucose is not always relevant to body weight (22). What is interesting in this data is that exendin-4 alone and in combination with BPA maintained blood glucose, serum insulin, lipid profiles, leptin, adiponectin and oxidative markers at the normal value. Our data also clearly showed that BPA influenced MDA increase in tissue and plasma, which is the final product of lipid peroxidation, and exendin-4 compensated these distractive effects. This was consistent with recent reports that revealed clear benefit of exendin-4 in the prevention of reactive oxygen species (ROS) production and mitochondrial dysfunction and increase in blood glucose level, which could increase the production of ROS and could participate in oxidative damages (23). It is possible to hypothesis that these conditions are due to either glucose regulatory and antioxidative effects of exendin-4.
Furthermore, this study showed that BPA caused a decrease in plasma adiponectin and increase in plasma leptin, while exendin-4 maintained these hormones at similar levels to control. This finding is in agreement with previous findings, which showed exendin-4 through direct effect on GLP-1 receptors and protein kinase A (PKA) pathway, increased adiponectin level (24, 25). The current experiment was consistent with that of Shabani et al. (2015), which revealed that reduced leptin is associated with decrease in blood glucose (26). Recent evidence suggests that leptin can stimulate ROS excessive formation through increase in nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase activation (27). Inflammation is the common basis for various diseases, and leptin contributes to the increase inflammatory cytokines (28, 29). Therefore, it can be assumed that exendin-4 via leptin modification may be influencial on decrease of inflammation, oxidative damages and metabolic disorders.
The most interesting finding was that exendin-4 improves hyperlipidemia in the treatment group. Many experiments revealed that there are correlations between oxidative stress and hyperglycemia and hyperlipidemia (30, 31). Also in 2015 kheirollah et al. showed that increase in insulin secretion and β cell reinforcement by control in glucose level and insulin sensitivity influenced the decrease in triglyceride (32). Therefore, the current study suggests that exendin-4 through β cell enhancement, glucose regulation and finally antioxidant properties modified lipid profile.
The present study provides additional evidence with respect to treatment effects of exendin-4 on hyperglycemia, hyperlipidemia and metabolic disorders induced by BPA, through improvement in adipocyte hormones and antioxidant defense system. These findings enhance our understanding of exendin-4, confirm previous findings, and provide additional evidence that highlight the importance of exendin-4 in metabolic disorders.
Acknowledgements
References
-
1.
Ahangarpour A, Zamaneh HT, Jabari A, Nia HM, Heidari H. Antidiabetic and hypolipidemic effects of Dorema aucheri hydroalcoholic leave extract in streptozotocin-nicotinamide induced type 2 diabetes in male rats. Iran J Basic Med Sci. 2014;17(10):808-14. [PubMed ID: 25729552].
-
2.
Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, et al. Low-level phenolic estrogen pollutants impair islet morphology and beta-cell function in isolated rat islets. J Endocrinol. 2012;215(2):303-11. [PubMed ID: 22946080]. https://doi.org/10.1530/JOE-12-0219.
-
3.
Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7(6):346-53. [PubMed ID: 21467970]. https://doi.org/10.1038/nrendo.2011.56.
-
4.
Nadal A, Alonso-Magdalena P, Ripoll C, Fuentes E. Disentangling the molecular mechanisms of action of endogenous and environmental estrogens. Pflugers Arch. 2005;449(4):335-43. [PubMed ID: 15517344]. https://doi.org/10.1007/s00424-004-1343-9.
-
5.
Aarab N, Lemaire-Gony S, Unruh E, Hansen PD, Larsen BK, Andersen OK, et al. Preliminary study of responses in mussel (Mytilus edilus) exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether. Aquat Toxicol. 2006;78 Suppl 1:S86-92. [PubMed ID: 16580744]. https://doi.org/10.1016/j.aquatox.2006.02.021.
-
6.
Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One. 2008;3(4). eee2069. [PubMed ID: 18446233]. https://doi.org/10.1371/journal.pone.0002069.
-
7.
Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50(4):625-31. [PubMed ID: 23636267]. https://doi.org/10.1007/s00592-013-0472-z.
-
8.
Mileva G, Baker SL, Konkle AT, Bielajew C. Bisphenol-A: epigenetic reprogramming and effects on reproduction and behavior. Int J Environ Res Public Health. 2014;11(7):7537-61. [PubMed ID: 25054232]. https://doi.org/10.3390/ijerph110707537.
-
9.
Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, et al. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia. 1999;42(1):45-50. [PubMed ID: 10027577]. https://doi.org/10.1007/s001250051111.
-
10.
Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117(2):77-88. [PubMed ID: 14700743].
-
11.
Young AA. 14. Glucagon-like peptide-1, exendin and insulin sensitivity. Insulin Resist Insulin Resist Syndrome. 2002;5:235.
-
12.
Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism. 2001;50(5):583-9. [PubMed ID: 11319721]. https://doi.org/10.1053/meta.2001.22519.
-
13.
Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751-60. [PubMed ID: 11078440].
-
14.
Sun F, Chai S, Li L, Yu K, Yang Z, Wu S, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res. 2015;2015:157201. [PubMed ID: 25688373]. https://doi.org/10.1155/2015/157201.
-
15.
Adachi T, Yasuda K, Mori C, Yoshinaga M, Aoki N, Tsujimoto G, et al. Promoting insulin secretion in pancreatic islets by means of bisphenol A and nonylphenol via intracellular estrogen receptors. Food Chem Toxicol. 2005;43(5):713-9. [PubMed ID: 15778011]. https://doi.org/10.1016/j.fct.2005.01.009.
-
16.
Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114(1):106-12. [PubMed ID: 16393666].
-
17.
Bodin J, Bolling AK, Samuelsen M, Becher R, Lovik M, Nygaard UC. Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice. Immunopharmacol Immunotoxicol. 2013;35(3):349-58. [PubMed ID: 23496298]. https://doi.org/10.3109/08923973.2013.772195.
-
18.
Batista TM, Alonso-Magdalena P, Vieira E, Amaral ME, Cederroth CR, Nef S, et al. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One. 2012;7(3). eee33814. [PubMed ID: 22470480]. https://doi.org/10.1371/journal.pone.0033814.
-
19.
Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009;117(10):1549-55. [PubMed ID: 20019905]. https://doi.org/10.1289/ehp.11342.
-
20.
Moghaddam HS, Samarghandian S, Farkhondeh T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol Mech Methods. 2015;25(7):507-13. [PubMed ID: 26376105]. https://doi.org/10.3109/15376516.2015.1056395.
-
21.
Rubin BS, Soto AM. Bisphenol A: Perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304(1-2):55-62. [PubMed ID: 19433248]. https://doi.org/10.1016/j.mce.2009.02.023.
-
22.
Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F, et al. Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure. PLoS One. 2013;8(5). eee64143. [PubMed ID: 23675523]. https://doi.org/10.1371/journal.pone.0064143.
-
23.
Raab EL, Vuguin PM, Stoffers DA, Simmons RA. Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1785-94. [PubMed ID: 19846744]. https://doi.org/10.1152/ajpregu.00519.2009.
-
24.
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001;507(2):142-6. [PubMed ID: 11684087].
-
25.
Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, et al. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun. 2009;390(3):613-8. [PubMed ID: 19850014]. https://doi.org/10.1016/j.bbrc.2009.10.015.
-
26.
Shabani Fard A, Shakerian S, Habibi AH, Faraji S. The Effects of 3000 Meters Swimming on Serum Level of Leptin and Glucose Under 2 Different Intensities: Continuous and Intermittent. Jentashapir J Health Res. 2015;6(6).
-
27.
Blanca AJ, Ruiz-Armenta MV, Zambrano S, Salsoso R, Miguel-Carrasco JL, Fortuno A, et al. Leptin Induces Oxidative Stress Through Activation of NADPH Oxidase in Renal Tubular Cells: Antioxidant Effect of L-Carnitine. J Cell Biochem. 2016;117(10):2281-8. [PubMed ID: 26918530]. https://doi.org/10.1002/jcb.25526.
-
28.
Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68(4):437-46. [PubMed ID: 11037963].
-
29.
Zhaleh M, Panahi M, Ghafurian Broujerdnia M, Ghorbani R, Ahmadi Angali K, Saki G. Role of phosphodiesterase inhibitor Ibudilast in morphine-induced hippocampal injury. J Inj Violence Res. 2014;6(2):72-8. [PubMed ID: 24121451]. https://doi.org/10.5249/jivr.v6i2.497.
-
30.
Lee YA, Cho EJ, Yokozawa T. Effects of proanthocyanidin preparations on hyperlipidemia and other biomarkers in mouse model of type 2 diabetes. J Agric Food Chem. 2008;56(17):7781-9. [PubMed ID: 18690694]. https://doi.org/10.1021/jf800639m.
-
31.
Arabmoazzen S, Sarkaki A, Saki G, Mirshekar MA. Antidiabetic effect of honey feeding in noise induced hyperglycemic rat: involvement of oxidative stress. Iran J Basic Med Sci. 2015;18(8):745-51. [PubMed ID: 26557962].
-
32.
Kheirollah A, Maghuli A, Dalvand P, Mohaghegh M, Barat T, Aberomand M. Effect of Hexagonal Water on Serum Glucose Level and Lipid Profile in Streptozotocin-Induced Diabetic Rats. Jentashapir J Health Res. 2015;6(5).