Abstract
Objectives:
CRP (C - reactive protein) is more often used to show hidden infections with the bacterial origin. CRP is most likely activated due to bacterial infection. The researcher in this study examined the effect of selenase on acute phase protein response.Methods:
In this clinical trial, all patients were men and women in the age range of 20 - 90 years old who were suffering from septic shock and the presence of positive bacterial cultures, Peoria, positive radiographic abscess, pneumonia, cellulites, gangrene, and infection in the presence of a urinary catheter that since the adoption of the proposal were admitted. To evaluate the mean level changes of plasma variables in the two groups, t-student test was used. The software used for statistical analysis was SPSS-18 and statistical values less than 0.05 were considered significant (P < 0.05).Results:
Laboratory findings where markers of the acute phase response were examined in this study include the increased platelet count and CRP. Both variables in the two groups were statistically significant (P < 0.05). The frequency of patients who had a platelet count below 150,000 dL in the case group (selenium) was 5 patients and in the control group (placebo) was 9 individuals. This decrease in platelet count among patients was in the 70,000 - 120,000 range. In the control group, elevated levels of CRP in test results was observed in the 13 patients and in the case group, this increase was seen in 8 patients.Conclusions:
Effect of the acute phase response for detection infection in patients with sepsis is still controversial. This study showed the positive effect of Selenium on patients who have suffered from septic shock.Keywords
1. Background
Acute phase proteins are the proteins that cause increase in leukocytes, fever, inflammation, and also a decrease in albumin rate. Proteins increase in these conditions: inflammation, sepsis, surgery complications, and trauma or tissue necrosis in response to the cytokines (IL1, IL6, and TNF). Acute phase reactive proteins include acid glycoprotein, haptoglobin, ceruloplasmin, fibrinogen, serum amyloid protein A, CRP (C- reactive protein), Factor VIII, ferritin, lipoprotein components of complement, and immunoglobulins (1). CRP (C - reactive protein) is more often used to show hidden infections with the bacterial origin, due to the fact that the bacterial infection is most likely to activate CRP.
Sepsis is a systemic deleterious host response to infections leading to severe sepsis (acute organ dysfunction secondary to documented or suspected infection) and septic shock (severe sepsis plus hypotension not reversed with fluid resuscitation) (2). As a result, the proinflammatory reaction can be uncontrolled as a cascade of complement activation, coagulation, arterial diffuse vasodilatation and capillary permeability altered at launch. Severe sepsis and septic shock are major healthcare problems affecting millions of people around the world each year. It is killing one in every four individuals (often more) and is increasing in incidence (2-7). CRP, due to the inhibitory effect, its destruction, fast answered to the acute phase stimulus, and has a wide concentration range and simplicity method of measurement in plasma make causes more use of it in assessment to intensity inflammation and treatment in infections (1).
Zimmerman et al. conducted the first study on the effect of selenium on 40 patients in 1997. They reported no significant effect for it in those who had severe systemic inflammatory response syndrome, sepsis, or septic shock (8). The selenium dependent glutathione-peroxidases (GPx) as well as thioredoxin reductases are important compounds responsible for the maintenance of the redox system in all cells including the immune-competent cells (9). Present knowledge stated that the activity of these enzymes is mainly regulated by the availability of selenium (10-13). During severe oxidative stress, like sepsis or septic shock, the requirement of selenium might be increased, as patients with systemic inflammatory activity (SIRS), sepsis, and septic shock exhibit low selenium and GPx activities (9). Darlow et al. in their study on preterm infants stated, a selenium supplementation decreased the morbidity (14, 15).
Reducing mortality in the intensive care unit (ICU) is still a major challenge (16, 17). It seems that due to the antioxidant properties of selenium and its other features, it can maintain and stabilize the vascular endothelium and normal body perfusion. Thus, using selenium compounds for treatment is more appropriate and impressive. This clinical trial has examined the effect of sodium selenite on the acute phase protein response and plasma variables in patient admitted in ICU in Tehran.
2. Methods
This randomized, double-blind clinical trial was conducted on the patients admitted to the ICU of Imam Reza hospital in Tehran with septic shock. The pilot study was done on a sample of 10 specimens and by using the formula n = Z2 * δ2/d2. The required sample size was 80 participants (40 patients in the placebo group and 40 patients in the treatment group). The specimens were sampled using simple random sampling. Method of allocating participants into two groups was based on the simple random sampling using the black and white cards. Confidence interval was 95%. All patients fulfilling the inclusion criteria were enrolled in the study. All participants were in age range of 20 to 90 years old suffering from septic shock, positive bacterial culture, Peoria, positive radiographic abscess, pneumonia, cellulites, gangrene, and infection in the presence of a urinary catheter since being admitted to the ICU. Informed consent was taken and if the patient was not conscious, signed consent was taken from his/her first degree relatives. The patients who had chronic liver disease and active gastrointestinal bleeding or were on dialysis, pregnant, and post cardio-pulmonary resuscitation were excluded from the trial. The study protocol was approved by ethics committee of AJA University of Medical Sciences. Data were collected by observation and recorded in a questionnaire. Questionnaire reliability by using Cronbach’s alpha coefficient (α = 0.83) and its validity was confirmed by professors of the department of anesthesiology. Anthropometric data such as age and gender were gathered and the amount of each studied variable in both groups were also recorded before starting the treatment. Every participant in the treatment group (case group) was administered 500 μg selenase twice daily for 10 days. In contrast, each participant of the control group was treated twice daily for a period of 10 days with placebo (normal saline). At the end of treatment, the studied variables were recorded again. Chi-square test was used to assess the status of two groups in respect of variables such as age and gender. Paired t-test was used to evaluate the mean level changes of plasma variables in the two groups. The statistical package for social sciences (SPSS) software version 18 was used for statistical analysis. P values less than .05 were considered significant.
3. Results
Of the 80 under study patients, 34 patients (42.5%) were males and 46 (57.5%) were female. According to Table 1, in both groups, no significant differences were found between demographic data (age, sex, and weight) (P > 0.05). The mean age of patients in the selenium group (case group) was 58.25 ± 17.6 and in the control group (normal saline) was 59.25 ± 16.4 years old. In addition, age range was between 85 - 22 years in the case group and 90 - 24 in the control group. There was no statistically significant difference between the groups (P > 0.05).
The Demographic Characteristics of Patients in the Two Study Groups
| Variables | The Frequency of Individuals in Groups According to Age Group | P Value | |
|---|---|---|---|
| Age Groups, y | Case Group | Control Group | |
| 20 - 30 | 3 | 2 | 0.311 |
| 31 - 40 | 3 | 2 | |
| 41 - 50 | 6 | 4 | |
| 51 - 60 | 10 | 12 | |
| 61 - 70 | 7 | 8 | |
| 71 - 80 | 6 | 5 | |
| 81 - 90 | 53 | 72 | |
| Sex ratio (female to male) | 19 to 21 | 17 to 23 | |
Frequency of the variables and individual characteristics such as the source of infection, pathogenicity factors, and co morbidities are listed in Table 2. As it is clear from Table 2 there was no statistically significant difference observed between the patients individual characteristics in the two groups (P > 0.05). Furthermore, according to Table 2, the prevalence of diabetes was higher than all other co morbidities diseases.
Compare the Characterization of Randomized Patients in Two Study Group
| Variable | Case Group (Selenium) | Control Group (Normal Salin) | P Value |
|---|---|---|---|
| Site of infection | 0.217 | ||
| Pneumonia | 9 | 8 | |
| Osteomyelitis | 5 | 3 | |
| Meningitis | 1 | 2 | |
| Peritonitis | 9 | 6 | |
| Gluteal abscess | 3 | 3 | |
| Narcotics injecting (Bacteremia) | 7 | 9 | |
| UTI (Urinary Tract Infection) | 9 | 9 | |
| Pathogen | 0.301 | ||
| Anaerobic | 10 | 12 | |
| Fungi (Candida and Aspergillus) 7 | 9 | ||
| Gram positive | 10 | 8 | |
| Gram negative | 13 | 11 | |
| Comorbidities | 0.208 | ||
| DM|(diabetes mellitus type 1) | 6 | 5 | |
| DM||(diabetes mellitus type 2) | 7 | 8 | |
| CVA (cerebrovascular accident) | 4 | 5 | |
| IHD (ischaemic heart disease) | 5 | 6 | |
| COPD (chronic obstetric pulmonary disease) | 9 | 10 | |
| HTN (hypertension) | 9 | 6 |
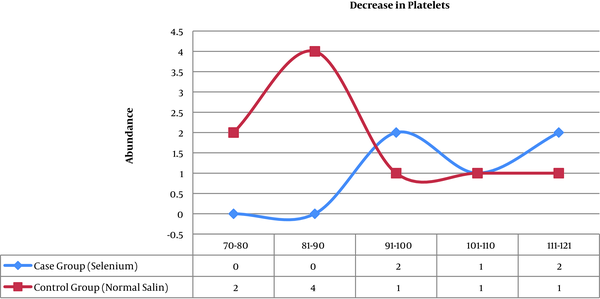
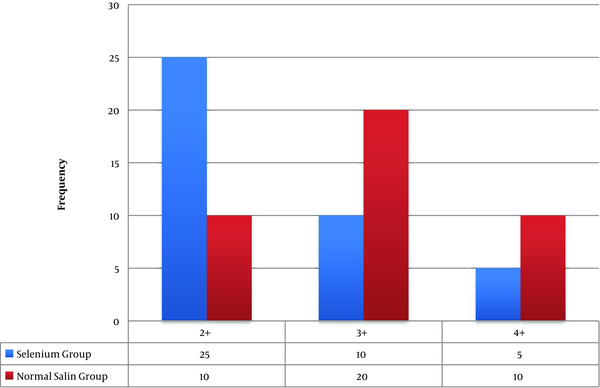
Other laboratory findings, that as markers of the acute phase response were examined in this study, include the increased platelet count and CRP. Both variables in the two groups were statistically significant. The frequency of patients who had a platelet count below 150,000 dL in the case group (selenium) was 5 patients and in the control group (placebo) was 9 people. This decrease in platelet count among patients was in the 70,000 - 120,000 range (Figure 1). In the control group (placebo), elevated levels of CRP in test results was observed in the 13 patients and in the case group (selenium), this increase was seen in 8 patients (Figure 2)
Compare platlate diagram in two groups (P = 0.024)
Compare CRP diagram in two groups (P = 0.002)
The number of days that patients require administration of catecholamines (norepinephrine) in the case group (selenium) was 4 patients and in the control group (placebo) was 9 patients and this difference was statistically significant (P < 0.05).
The number of days that the patients were oliguric, in selenium group was 3 days and in the placebo group was 5 days.
4. Discussion
In the present study, in the acute phase responses of the two groups, statistically significant differences were observed. Berger, in 2008, assessed the impact of rapid administration of antioxidant supplements in the early hours of ICU admission on limb function in patients with severe disease (surgery Trauma large and subarachnoid hemorrhage), they divided their patients into two groups, groups who receiving supplemental antioxidants (AOX) (including vitamins B and C, zinc oxide and selenium) and the placebo group. They treated patients for 5 days. They observed that the levels of the inflammatory marker in the group that had received the antioxidant supplements were much lower than the placebo group. Also CRP levels reduced in those who had received antioxidant very rapidly (18). In the Matthias study, CRP level in the group who had received selenium was lower than the placebo (9). Almaa Salma stated in their study that plasma selenium levels were inversely associated with CRP, Procalcitonin, and interleukin-6 (19). The results of these studies are quite similar to the present investigation.
The effect of the acute phase response for detection infection in patients with sepsis is still controversial and a number of studies of the effectiveness use of procalcitonin level or other acute phase response (such as CRP), in order to tailor the differentiation differentiate sepsis from other causes of acute inflammatory, generalized inflammation was not proved. Therefore, there was no advice on the use of these markers for application in differentiating between serious infections and other acute inflammatory conditions (3, 20-22).
In our study, the prevalence of patients who had a platelet count below 150,000 per dl in the case group (selenium) was less than in the control group (placebo). Some studies have stated that patients with thrombocytopenia were associated with a poor outcome (23-25). The results of these studies were consistent with our results.
Forceville et al. examined the effects of high doses of selenium on patients with septic shock in their multicenter study, patients were evaluated in two groups: treated with selenium (for 10 days) and the placebo group. The results of his study showed that the mean platelet in patients who have received selenium was less than the placebo group, however, this difference was not statistically significant (26). Some studies have suggested that male patients with coronary heart disease and platelet levels are inversely associated with plasma levels of selenium linked (27, 28). The results of these studies were consistent with the findings of our study. Use of catecholamines to preserve and sustain life and tissue perfusion is required when confrontation with life threatening hypotension, even when hypovolemia is still not resolved. Thus, there may be some patients achieving minimum perfusion pressure and maintaining enough flow that need to have vasopressor therapy (29, 30). Studies have shown that administration of norepinephrine to restore MAP at least 65 mmHg protected tissue perfusion (29, 30). In this study the number of days that patients require administration of catecholamines (norepinephrine) in the control group was less than the placebo group and this difference was statistically significant. In the study by Forceville et al. the need to norepinephrine catecholamines in the control group (selenium) was 13 patients and in the control group (placebo) was 19 patients. The results of his study indicate the clinical efficacy of selenium in reducing the need for norepinephrine in patients; however, this difference was not statistically significant (26). Forceville results was consistent with the results of our study.
4.1. Conclusions
Effect of the acute phase response for detection infection in patients with sepsis is still controversial. The results of the present study showed the positive effect of Selenium on patients who have suffered from septic shock and also its effect on reducing the rate of CRP in patient with sepsis. That while the conduct of clinical trials on the efficacy of different doses of selenium, as well as ambiguities and questions to determine the best dose and the administration of what they do. Although the clinical treatment of patients with septic shock is a dynamic and evolving process (20), new clinical methods with different approaches to the treatment of sepsis and septic shock, as well as international guidelines on the treatment and survival of these patients have played a significant role. However, there is a need to conduct further multicenter clinical trials in this field and integration and combining the clinical knowledge and experience to generalize the results of evidence-based clinical studies.
4.2. Study Limitations
The main problem is the study of factors affecting High Over the course of the disease in patients with septic shock was considered as a confounding variable. We had so many things in the exclusion criteria. To enhance the effect of the drug on the disease, the process is more objective review.
Acknowledgements
References
-
1.
Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805-12. [PubMed ID: 12813013]. [PubMed Central ID: PMC161431]. https://doi.org/10.1172/JCI18921.
-
2.
Arupconsult. Acute Phase Inflammatory Proteins - Acute Phase Reactants. 2015. Available from: http://www.arupconsult.com/index.html.
-
3.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637. [PubMed ID: 23353941]. https://doi.org/10.1097/CCM.0b013e31827e83af.
-
4.
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-54. [PubMed ID: 12700374]. https://doi.org/10.1056/NEJMoa022139.
-
5.
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-10. [PubMed ID: 11445675].
-
6.
Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244-50. [PubMed ID: 17414736]. https://doi.org/10.1097/01.CCM.0000261890.41311.E9.
-
7.
Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003;31(3):946-55. [PubMed ID: 12627010]. https://doi.org/10.1097/01.CCM.0000057403.73299.A6.
-
8.
Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care. 2004;8(4):222-6. [PubMed ID: 15312201]. [PubMed Central ID: PMC522859]. https://doi.org/10.1186/cc2917.
-
9.
Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31(3):327-37. [PubMed ID: 15605227]. https://doi.org/10.1007/s00134-004-2522-z.
-
10.
Angstwurm MWA, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock*. Critical Care Medicine. 2007;35(1):118-26. https://doi.org/10.1097/01.ccm.0000251124.83436.0e.
-
11.
Schomburg L, Schweizer U, Kohrle J. Selenium and selenoproteins in mammals: extraordinary, essential, enigmatic. Cell Mol Life Sci. 2004;61(16):1988-95. [PubMed ID: 15316649]. https://doi.org/10.1007/s00018-004-4114-z.
-
12.
Burk RF, Hill KE, Motley AK. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J Nutr. 2003;133(5 Suppl 1):1517S-20S. [PubMed ID: 12730456]. https://doi.org/10.1093/jn/133.5.1517S.
-
13.
Birringer M, Pilawa S, Flohe L. Trends in selenium biochemistry. Nat Prod Rep. 2002;19(6):693-718. [PubMed ID: 12521265].
-
14.
Dodig S, Cepelak I. The facts and controversies about selenium. Acta Pharm. 2004;54(4):261-76. [PubMed ID: 15634611].
-
15.
Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003;(4). CD003312. [PubMed ID: 14583967]. https://doi.org/10.1002/14651858.CD003312.
-
16.
Darlow BA, Winterbourn CC, Inder TE, Graham PJ, Harding JE, Weston PJ, et al. The effect of selenium supplementation on outcome in very low birth weight infants: a randomized controlled trial. The New Zealand Neonatal Study Group. J Pediatr. 2000;136(4):473-80. [PubMed ID: 10753245].
-
17.
Sligl WI, Milner DJ, Sundar S, Mphatswe W, Majumdar SR. Safety and efficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin Infect Dis. 2009;49(1):93-101. [PubMed ID: 19489712]. https://doi.org/10.1086/599343.
-
18.
Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am J Respir Crit Care Med. 2012;185(2):133-9. [PubMed ID: 21680949]. https://doi.org/10.1164/rccm.201011-1897CI.
-
19.
Berger MM, Soguel L, Shenkin A, Revelly JP, Pinget C, Baines M, et al. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care. 2008;12(4):R101. [PubMed ID: 18687132]. [PubMed Central ID: PMC2575590]. https://doi.org/10.1186/cc6981.
-
20.
Salama A, Sakr Y, Reinhart K. The role of selenium in critical illness: basic science and clinical implications. Indian JCrit Care Med. 2007;11(3):127.
-
21.
Giamarellos-Bourboulis EJ, Giannopoulou P, Grecka P, Voros D, Mandragos K, Giamarellou H. Should procalcitonin be introduced in the diagnostic criteria for the systemic inflammatory response syndrome and sepsis? J Crit Care. 2004;19(3):152-7. [PubMed ID: 15484175].
-
22.
Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34(7):1996-2003. [PubMed ID: 16715031]. https://doi.org/10.1097/01.CCM.0000226413.54364.36.
-
23.
Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7(3):210-7. [PubMed ID: 17317602]. https://doi.org/10.1016/S1473-3099(07)70052-X.
-
24.
Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357-65. [PubMed ID: 15547163]. https://doi.org/10.1001/jama.292.19.2357.
-
25.
Murphy DJ, Hope PL, Johnson A. Neonatal risk factors for cerebral palsy in very preterm babies: case-control study. BMJ. 1997;314(7078):404-8. [PubMed ID: 9040385]. [PubMed Central ID: PMC2125924].
-
26.
Jafari AH, Bilan N, Taghizadieh A, Tahmasebi S, Navidi P. Factors that affect the outcomes in children with decompensated shock. Med J Tabriz Univ Med Sci. 2012;34(2):26-30.
-
27.
Forceville X, Laviolle B, Annane D, Vitoux D, Bleichner G, Korach JM, et al. Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care. 2007;11(4):R73. [PubMed ID: 17617901]. [PubMed Central ID: PMC2206523]. https://doi.org/10.1186/cc5960.
-
28.
Neve J. Selenium as a risk factor for cardiovascular diseases. J Cardiovasc Risk. 1996;3(1):42-7. [PubMed ID: 8783029].
-
29.
Salonen JT, Salonen R, Seppanen K, Kantola M, Parviainen M, Alfthan G, et al. Relationship of serum selenium and antioxidants to plasma lipoproteins, platelet aggregability and prevalent ischaemic heart disease in Eastern Finnish men. Atherosclerosis. 1988;70(1-2):155-60. [PubMed ID: 3258519].
-
30.
Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32(9):1928-48. [PubMed ID: 15343024].