Abstract
Context:
Periodontal disease is a complication of diabetes mellitus. Both periodontal disease and diabetes mellitus stimulate the release of proinflammatory cytokines. The aim of the present study was to evaluate the salivary and serum levels of interleukin (IL-6) and IL-8 levels in type II diabetic patients with periodontal disease.Evidence Acquisition:
The present study is a narrative review. A literature review was conducted using the electronic databases including Web of Science, PubMed, Scopus, Google Scholar as well as Persian databases such as SID, Magiran, and IranMedex from 2005 to 2019, particularly the last 10 years. The appropriate keywords were searched, including “Diabetes Mellitus”, “IL-6”, “IL-8”, Periodontal Disease”, “Saliva”, and “Serum”.Results:
The present study analyzed four articles from the case-control series, including 42 to 90 patients. The results showed that the level of salivary concentration of IL-6 was increased in patients with periodontal disease with/without diabetes mellitus. A marginally statically significant correlation was found in salivary and serum levels of IL-6 after applying spearman’s nonparametric test. However, the relevant serum analysis showed only a minor influence of type II diabetes and periodontal disease on IL-6 serum levels. There was no significant difference between the periodontitis patients and IL-6 and IL-8 serum levels. A positive correlation was found between glycemic control and the severity of periodontal disease.Conclusions:
The intensity of periodontal disease was high in patients with type II diabetes, underlining the need for special oral health care for these patients. The level of salivary IL-6 can be considered as a main biomarker in the diagnosis of diabetes and periodontal disease. The serum levels of the IL-6 and IL-8 showed no significant difference in patients with periodontitis.Keywords
1. Context
Periodontal disease (PD) is one of the most common infectious diseases among humans. It is a bacterial inflammatory condition of the gum and periodontal tissues surrounding the teeth (1). Dental plaque is a predisposing factor in the destruction of the teeth and periodontal tissues (2). It is associated with development and reproduction of microorganisms, epithelial cells leukocytes, and macrophages (3). Periodontal tissue demolition is interceded by locally produced proinflammatory cytokines in response to the bacteria flora and its products (4).
The main risk factors for periodontitis are tobacco, smoking, and diabetes (5). There is a close link between periodontal disease and diabetes mellitus (DM). In other words, patients with type II diabetes are more likely at high risk (2.81 times) of clinical attachment loss than healthy individuals (6). In addition, glycemic control can affect the PD severity. People who have type II diabetes have an increased risk of alveolar bone resorption compared to well-controlled diabetes (7).
Strong evidence for a relationship between DM and PD has led some researchers to classify PD as a common complication of diabetes. However, the exact mechanisms of these interactions are not yet fully understood (8). There is a change in monocyte/macrophage of patients with type II diabetes, which results in the overproduction of proinflammatory cytokines in response to periodontal pathogens (9), which could exacerbate the pathogenesis of PD (10). The increased level of cytokines such as interleukin-6 (IL-6) has been observed in diabetic patients, so a higher level of serum IL-6 has been shown to be associated with diabetes (11, 12). IL-6 is a multifunctional cytokine produced by a specific variety of cells, including macrophages, neutrophils, and endothelial cells (13). Interleukin-8 (IL-8) is a chemokine that acts as a key role in the conscription of neutrophils during healing. Its level was shown to be tightly linked to increased susceptibility of PD (14). IL-8 is associated with the initiation and increase of acute inflammatory reaction; it is secreted by a number of cell types in reaction to inflammatory stimuli (15). The increased cytokine level in the salivary and gingival crevicular fluid (GCF) of the patients with PD are typical markers of the initiation and maintenance of these pathogens. Saliva is considered as a source of non-invasive examination of metabolism and the abolition of many drugs (16). Despite the contradictory outcomes, studies have suggested that some markers such as IL-6 and interleukin-1beta (IL-1B) are associated with insulin resistance and glycemic control (17).
IL-6 and IL-8 have been characterized as a fundamental marker of immune response and critical modulator in the initiation, progression, and/or suppression of chronic periodontitis (18).
The aim of the present review study was to evaluate the salivary and serum levels of IL-6 and IL-8 in type II diabetic patients with periodontal disease.
2. Evidence Acquisition
2.1. Search Strategy
This study is a narrative review conducted via electronic databases such as Web of Science, PubMed, Scopus, Google Scholar as well as Persian databases such as SID, Magiran, IranMedex from 2000 to 2019, particularly the last 10 years. The appropriate keywords were searched, including “Diabetes Mellitus”, “Interleukin-6”, “IL-6”, “Interleukin-8”, “IL-8”, “Periodontal Disease”, “Saliva”, and “Serum” in the “Title”, “Abstract”, or “Keywords”, until Dec 2019. The relevant data were reviewed, and the eligible studies were selected for the final analyses. Two reviewers were independently confirmed eligible articles.
2.2. The Inclusion Criteria
1) Case-control, human clinical trial, and prospective studies.
2) Studies on salivary and serum levels of IL-6 and IL-8 in patients with chronic periodontal disease and type II diabetes.
2.3. Exclusion Criteria
1) The case reports and studies on the serum IL-6 and IL-8 of the GCF and gingival biopsies of the patients with diabetes and PD.
2) Studies that investigated the effects of other cytokines on the PD and type II diabetes
3) Studies of the IL-6 and IL-8 levels in the patients with either type II diabetes or PD.
4) Duplicate manuscripts.
The following data were extracted from the predefined list: first author, year, design, study period, age, intervention, control, number of participants in intervention and control groups, adverse effect, and outcome measurements.
3. Results
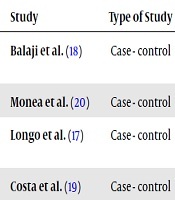
The present study examined the abstracts and full texts of the articles and finally four articles were included about the salivary and serum levels of IL-6 and IL-8 in type II diabetic patients with PD. Tables 1 and 2 show a summary of the results of research studies. The designs of all the selected articles were case-control studies. The sample size included 42 to 90 patients in each study.
Salivary and Serum Levels of IL-6 and IL-8 in Patients with Type II Diabetes with PD from the Studied Articles
| Study | Name of Study | Conclusions |
|---|---|---|
| Balaji et al. (18) | Evaluating the Salivary concentrations of IL-6 in periodontitis patients with T2D. | Salivary IL-6 level is a key biomarker in the diagnosis of periodontitis and diabetes. |
| Costa et al. (19) | Determining the levels of IL-6 in saliva in patients with diabetes with PD. | Similar to the study by Balagi et al. (17). |
| Monea et al. (20) | Determining saliva and serum levels of IL-6 in periodontal patients, with or without T2DM. | The level of IL-6 in the saliva was higher in T2D subjects with PD than the systemically healthy subjects with PD. Serum IL-6 levels revealed only a minor impact of the T2D and PD. |
| Longo et al. (17) | Evaluating serum levels of IL-6 and IL-8 in T2D with CP. | Although periodontitis was more severe in patients with diabetes, the serum level of the investigated inflammatory markers was not different among patients with PD |
Characteristic of the Studied Articles
| Study | Type of Study | Sample Size | Groups | Type of Cytokines | Sample Location |
|---|---|---|---|---|---|
| Balaji et al. (18) | Case - control | 80 | H (n = 20); Untreated Periodontitis (n = 20); Diabetes Mellitus type IIb (n = 20); UPD + DM (n = 20) | IL-6 | Saliva |
| Monea et al. (20) | Case - control | 62 | H + PD (n = 21); H (n = 21); DM + PD (n = 20) | IL-6 | Saliva and Serum |
| Longo et al. (17) | Case - control | 42 | DMI + PD (n = 10); DMA + PD (n = 10); DM (n = 10); PD (n = 6); H (n = 6) | IL-6 and IL-8 | Serum |
| Costa et al. (19) | Case - control | 90 | UPD (n = 24); DM (n = 20); UPD + DM (n = 24); H (n = 22) | IL-6 | Saliva |
In the study of Balaji et al. (18), the saliva samples were collected from 80 patients and divided into four equal groups (Table 2). Salivary concentrations of the IL-6 were assessed by Human Quantikine ELISA kit. HbA1c levels were evaluated for metabolic assessment in type II diabetic patients and a healthy periodontium (DM) and patients with diabetes and periodontitis. HbA1c was measured and reported as percentages. The level of HbA1c test was in the normal range (< 6%). The results showed that the peak value of IL-6 concentrations was found in the uncontrolled type II diabetes and periodontitis group, and the control group showed the lowest level. The concentration of IL-6 was increased in subjects with untreated periodontitis (UPD) and diabetic patients with healthy periodontium. The DM + UPD group had HbA1c values higher than the other groups. The two-way relationship between DM and chronic periodontitis was confirmed (18).
Costa et al. (19) examined the salivary interleukin‐6, matrix metalloproteinase‐8, and osteoprotegerin in patients with PD and diabetes. The results of this study were consistent with the results of the Balaji et al. study.
Monea et al. (20) studied the salivary and serum of TNF-α and IL-6 levels in periodontal patients with/without type 2 diabetes mellitus (T2D) (Table 2). Un-stimulated salivary samples were collected from the participants. Venous blood samples were acquired by venipuncture via a sufficient closed system sample. Salivary and serum IL-6 levels were analyzed with the ELISA- sandwich method. Similarly, they determined the level of blood glucose and HbA1c in each diabetic patient. The diabetic patients were all formerly treated with oral antidiabetic drugs. The results of the study showed that the levels of salivary IL-6 were highly elevated in T2D patients with PD (91.12 ± 36.92 pg/mL) compared to systemically healthy periodontopathic patients (14.09 ± 7.28 pg/mL) and healthy controls (7.41 ± 2.46 pg/mL), with a high statistical significance (P < 0.0001). The level of serum IL-6 in parodontopathy diabetics was considerably increased (P = 0.0144) in comparison with systemically healthy periodontopathic patients and controls (P < 0.0001). A marginally statically substantial association was found between IL-6 serum and salivary levels after applying spearman’s nonparametric test. No correlations were found among serum IL-6 levels, glycemia, and HbA1c (20).
Longo et al. (17) evaluated the serum levels of inflammatory markers in type II diabetic patients with chronic periodontitis. They selected a total of 42 individuals with type II diabetes (T2D) and periodontal disease (PD) and divided them into two groups: satisfactory glycemic control (HbA1c < 8.0%), and poor glycemic control (HbA1c > 8.0%). The other three groups included T2D individuals without periodontitis, non- diabetic with periodontitis, and nondiabetic without periodontitis. Glycemic control was evaluated by serum concentration of HbA1c. The serum levels of IL-8 and IL-6 were measured by the ELISA method. A positive correlation was found between glycemic control and the severity of PD. No significant difference was found between IL-6 and IL-8 serum levels in the studied groups (17).
4. Discussion
Periodontal disease (PD) and diabetes mellitus (DM) are common and multifactorial diseases with high prevalence worldwide (21). The relationship between PD and DM is bidirectional (DM
There is a potential correlation between DM and PD, which encompasses a broad axis of inflammation, specific immune cell phenotype, serum lipid levels, and tissue homeostasis (24). Biomarkers are often studied in three areas, including early diagnosis of disease, severity of disease, and effective therapeutic methods. The serum, GCF, and saliva are appropriate sites for collecting biological samples (25). Furthermore, biopsy from the gingival tissue is often used to determine the level of the biomarkers (26). Dental investigations have developed a new technology to help dentists identify the source of pathologic changes (27). Saliva is used as a diagnostic tool in medicine and dentistry due to the easy collection method and storage and containing locally-produced microbial and host response mediators. Salivary biomarkers serve as a valuable diagnostic tool for the detection of periodontitis (20). The present review study provides a novel insight of salivary and serum levels of IL-6 and IL-8 levels in type II diabetic patients with periodontal disease.
Reviewing the studied articles specified that the concentration of salivary IL-6 was increased in patients with PD with/without diabetes. The IL-6 is a proinflammatory cytokine that directs the transition of inflammation from severe to chronic via shifting the nature of leukocyte infiltration. Also, it stimulates T and B cells and induces antibody formation and stimulates chronic inflammatory reaction (28, 29).
In addition, IL-6 inspires osteoclast activity and bone resorption (30). Periodontitis and diabetes are chronic inflammatory diseases that increase the salivary IL-6 level. Periodontitis triggers the systemic and local immune-inflammatory response via increasing the expression of IL-6, which further contributes to bone loss by induction of bone resorption (31).
Balaji et al. reported a positive association between poor metabolic control and periodontitis (19). However, the respective serum analysis showed only a minor influence of T2D and PD on IL-6 levels (20). This ratifies the hypothesis that DM acts as a cofactor in the initiation and evolution of PD. Moreover, Moneal et al. showed the increased levels of serum IL-6 (i.e., the salivary IL-6 in the diabetic patients with PD) were significantly higher than the systemically healthy subjects with PD and controls. This result supports the hypothesis that the PD associated-inflammation is more severe in patients with T2D than the systemically healthy individuals (32).
The present review showed that there was no significant difference in the serum level of IL-6 and IL-8 among the studied articles. The role of locally produced IL-6 has been widely reported in the periodontitis pathogenesis (33, 34). The high levels of IL-6 were realized in symptomatic large periodontal lesions rather than asymptomatic minor lesions (35). The overexpression of cytokines in PD mainly originates from the patients without diabetes and patients with poorly controlled diabetes (35). The literature shows conflicting results regarding the periodontal treatment’s effect on peak serum level of IL-6, indicating a propensity toward an increase or decrease of serum levels in T2D patients (36, 37). It should be noted that the individuals with T2D were treated with insulin, which may induce a low level of inflammatory biomarkers such as IL-6 (38) and lessen the high level of inflammation induced by periodontitis (8).
In the Longo et al. study, there was no significant difference between IL-6 and IL-8 serum levels in the studied groups; however, the previous data revealed that both were overexpressed in the gingival tissue with chronic periodontitis. IL-8 level was significantly increased in the gingival fluid of patients with PD. However, T2D subjects presented a significantly lower level of IL-8 in the gingival fluid when compared to healthy subjects (18).
Furthermore, the IL-8 serum level tended to decrease after periodontal therapy in T2D patients (36). It should be noted that serum analysis provided a valued analysis of the inflammatory markers in the studied subject (18).
Given the limitations of the studied articles and studying the cumulative effect of periodontal conditions on serum inflammatory biomarkers in patients with T2D, future research would benefit from the use of strict glycemic control and larger sample size.
5. Conclusions
The intensity of periodontal disease was high in patients with type II diabetes, underlining the need for special oral health care for these patients. The level of salivary IL-6 can be considered as the main biomarker in the diagnosis of diabetes and periodontal disease. The serum levels of the IL-6 and IL-8 did not show a significant difference in patients with periodontitis.
References
-
1.
Jahanghirnejad M, Babadi F, Safikhani E, Hemmati AA, Amiri Y. Comparison of the Effects of Chlorhexidine Mouthwash with Jaftex on Periodontal Index. Jentashapir Journal of Health Research. 2017;9(1). https://doi.org/10.5812/jjhr.11981.
-
2.
Babadi F, Amin M, Sharafi N, Saki M. Comparison of the Antibacterial Effects of Jaftex Herbal Mouthwash with Matrica and Persica on Streptococcus mutans, Streptococcus sanguinis, Streptococcus salivarius and Lactobacillus casei. Journal of Research in Medicinal and Dental Science. 2018;6(5):349-54.
-
3.
Yoshioka H, Yoshimura A, Kaneko T, Golenbock DT, Hara Y. Analysis of the activity to induce toll-like receptor (TLR)2- and TLR4-mediated stimulation of supragingival plaque. J Periodontol. 2008;79(5):920-8. [PubMed ID: 18454672]. https://doi.org/10.1902/jop.2008.070516.
-
4.
Warnakulasuriya S, Dietrich T, Bornstein MM, Peidró EC, Preshaw PM, Walter C, et al. Oral health risks of tobacco use and effects of cessation. International dental journal. 2010;60(1):7-30. https://doi.org/10.1922/IDJ_2532Warnakulasuriya24.
-
5.
Mesia R, Gholami F, Huang H, Clare-Salzler M, Aukhil I, Wallet SM, et al. Systemic inflammatory responses in patients with type 2 diabetes with chronic periodontitis. BMJ Open Diabetes Res Care. 2016;4(1). e000260. [PubMed ID: 27651910]. [PubMed Central ID: PMC5020743]. https://doi.org/10.1136/bmjdrc-2016-000260.
-
6.
Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Severe Periodontitis and Risk for Poor Glycemic Control in Patients with Non-Insulin-Dependent Diabetes Mellitus. Journal of Periodontology. 1996;67(10s):1085-93. https://doi.org/10.1902/jop.1996.67.10s.1085.
-
7.
Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329-34. [PubMed ID: 8422804]. https://doi.org/10.2337/diacare.16.1.329.
-
8.
Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68(2):127-35. [PubMed ID: 9058329]. https://doi.org/10.1902/jop.1997.68.2.127.
-
9.
Crook M. Type 2 diabetes mellitus: a disease of the innate immune system? An update. Diabet Med. 2004;21(3):203-7. [PubMed ID: 15008827]. https://doi.org/10.1046/j.1464-5491.2003.01030.x.
-
10.
Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4-7. [PubMed ID: 14698275].
-
11.
Schmidt MI, Duncan BB, Sharrett A, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. The Lancet. 1999;353(9165):1649-52. https://doi.org/10.1016/s0140-6736(99)01046-6.
-
12.
Khosravi R, Ka K, Huang T, Khalili S, Nguyen BH, Nicolau B, et al. Tumor necrosis factor- alpha and interleukin-6: potential interorgan inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediators Inflamm. 2013;2013:728987. [PubMed ID: 24068858]. [PubMed Central ID: PMC3771422]. https://doi.org/10.1155/2013/728987.
-
13.
Figueredo CM, Gustafsson A. Increased amounts of laminin in GCF from untreated patients with periodontitis. J Clin Periodontol. 2000;27(5):313-8. [PubMed ID: 10847534]. https://doi.org/10.1034/j.1600-051x.2000.027005313.x.
-
14.
Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559-64. [PubMed ID: 7964163].
-
15.
Azevedo LR, De Lima AAS, Machado MÂN, Grégio AMT, de Almeida PDV. Saliva Composition and Functions: A Comprehensive Review. The Journal of Contemporary Dental Practice. 2008;9(3):72-80. https://doi.org/10.5005/jcdp-9-3-72.
-
16.
Mealey BL, Oates TW, American Academy of P. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289-303. [PubMed ID: 16881798]. https://doi.org/10.1902/jop.2006.050459.
-
17.
Longo PL, Artese HP, Rabelo MS, Kawamoto D, Foz AM, Romito GA, et al. Serum levels of inflammatory markers in type 2 diabetes patients with chronic periodontitis. J Appl Oral Sci. 2014;22(2):103-8. [PubMed ID: 24676580]. [PubMed Central ID: PMC3956401]. https://doi.org/10.1590/1678-775720130540.
-
18.
Balaji A, Chandrasekaran SC, Subramanium D, Fernz AB. Salivary Interleukin-6 - A pioneering marker for correlating diabetes and chronic periodontitis: A comparative study. Indian J Dent Res. 2017;28(2):133-7. [PubMed ID: 28611321]. https://doi.org/10.4103/ijdr.IJDR_167_14.
-
19.
Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, et al. Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010;81(3):384-91. [PubMed ID: 20192865]. https://doi.org/10.1902/jop.2009.090510.
-
20.
Monea A, Mezei T, Popsor S, Monea M. Oxidative Stress: A Link between Diabetes Mellitus and Periodontal Disease. Int J Endocrinol. 2014;2014:917631. [PubMed ID: 25525432]. [PubMed Central ID: PMC4265692]. https://doi.org/10.1155/2014/917631.
-
21.
Borgnakke WS, Ylostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013;40 Suppl 14:S135-52. [PubMed ID: 23627324]. https://doi.org/10.1111/jcpe.12080.
-
22.
Díaz-Romero RM, Ovadía R. Diabetes and periodontal disease: a bidirectional relationship. Med Biol. 2007;14(1):6-9.
-
23.
Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6(1):125-37. [PubMed ID: 11887455]. https://doi.org/10.1902/annals.2001.6.1.125.
-
24.
Jaedicke KM, Preshaw PM, Taylor JJ. Salivary cytokines as biomarkers of periodontal diseases. Periodontol 2000. 2016;70(1):164-83. [PubMed ID: 26662489]. https://doi.org/10.1111/prd.12117.
-
25.
Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20(1):59-68. [PubMed ID: 16389170]. https://doi.org/10.1016/j.jdiacomp.2005.05.006.
-
26.
Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, et al. Current developments in salivary diagnostics. Biomark Med. 2010;4(1):171-89. [PubMed ID: 20387312]. [PubMed Central ID: PMC2857781]. https://doi.org/10.2217/bmm.09.68.
-
27.
Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63(5):321-9. [PubMed ID: 16640655]. https://doi.org/10.1111/j.1365-3083.2006.01750.x.
-
28.
Doganci A, Sauer K, Karwot R, Finotto S. Pathological Role of IL-6 in the Experimental Allergic Bronchial Asthma in Mice. Clinical Reviews in Allergy & Immunology. 2005;28(3):257-70. https://doi.org/10.1385/criai:28:3:257.
-
29.
Cutler CW, Machen RL, Jotwani R, Iacopino AM. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J Periodontol. 1999;70(11):1313-21. [PubMed ID: 10588494]. https://doi.org/10.1902/jop.1999.70.11.1313.
-
30.
Mihara M, Moriya Y, Kishimoto T, Ohsugi Y. Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor. Br J Rheumatol. 1995;34(4):321-5. [PubMed ID: 7788145]. https://doi.org/10.1093/rheumatology/34.4.321.
-
31.
Azuma MM, Samuel RO, Gomes-Filho JE, Dezan-Junior E, Cintra LT. The role of IL-6 on apical periodontitis: a systematic review. Int Endod J. 2014;47(7):615-21. [PubMed ID: 24224782]. https://doi.org/10.1111/iej.12196.
-
32.
Chapple IL, Genco R, Working group 2 of joint E. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40 Suppl 14:S106-12. [PubMed ID: 23627322]. https://doi.org/10.1111/jcpe.12077.
-
33.
Nibali L, Fedele S, D'Aiuto F, Donos N. Interleukin-6 in oral diseases: a review. Oral Dis. 2012;18(3):236-43. [PubMed ID: 22050374]. https://doi.org/10.1111/j.1601-0825.2011.01867.x.
-
34.
Venza I, Visalli M, Cucinotta M, De Grazia G, Teti D, Venza M. Proinflammatory gene expression at chronic periodontitis and peri-implantitis sites in patients with or without type 2 diabetes. J Periodontol. 2010;81(1):99-108. [PubMed ID: 20059422]. https://doi.org/10.1902/jop.2009.090358.
-
35.
Correa FO, Goncalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol. 2010;37(1):53-8. [PubMed ID: 19968741]. https://doi.org/10.1111/j.1600-051X.2009.01498.x.
-
36.
Sun WL, Chen LL, Zhang SZ, Wu YM, Ren YZ, Qin GM. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med. 2011;50(15):1569-74. [PubMed ID: 21804283]. https://doi.org/10.2169/internalmedicine.50.5166.
-
37.
Beisswenger PJ, Brown WV, Ceriello A, Le NA, Goldberg RB, Cooke JP, et al. Meal-induced increases in C-reactive protein, interleukin-6 and tumour necrosis factor alpha are attenuated by prandial + basal insulin in patients with Type 2 diabetes. Diabet Med. 2011;28(9):1088-95. [PubMed ID: 21517955]. [PubMed Central ID: PMC3178784]. https://doi.org/10.1111/j.1464-5491.2011.03324.x.
-
38.
Mao XM, Liu H, Tao XJ, Yin GP, Li Q, Wang SK. Independent anti-inflammatory effect of insulin in newly diagnosed type 2 diabetes. Diabetes Metab Res Rev. 2009;25(5):435-41. [PubMed ID: 19405039]. https://doi.org/10.1002/dmrr.968.