Abstract
Background:
Xylene is widely used in the industry and medical technology as a solvent, besides occupational exposure. It has the potential to be toxic in humans and animals.Objectives:
The present study was carried out to determine the protective effects of buffalo’ milk (BM) against xylene-induced liver and kidney damage.Methods:
A total 42 male rats were randomly assigned into 7 groups. The rats in group I was the control . Groups II, III and IV were received 1 mL distilled water ten minutes prior to administration of xylene (X) at doses of 0.81, 0.162 and 0.324 mmole/kg. Rats in groups of V,VI and VII were received 1mL buffalo’s milk 10 minutes prior to administration of X ( ip) at doses of 0.81, 0.162 and 0.324 mmole/kg. The experiment repeated for 7 consecutive days. 24 hours after last administrations, all animals were killed with an overdose of sodium pentobarbital. The blood was then collected for determination of biochemical tests including alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),blood urea nitrogen (BUN),creatinine (CR),catalase (CAT),superoxide dismutase (SOD) and glutathione (GSH). The liver and kidney tissues were removed, fixed and processed for light microscopy.Results:
Biochemical analyses indicated a significant decline in the activities of SOD, CAT and GSH level and markedly increased ALT, AST, ALP, BUN, CR when compared to those in non-treated rats (control). Dose-dependent injuries in rat liver and kidneys were also observed in xylene treated rats. Buffalo’s milk protected all biochemical parameters against xylene-induced toxicity. It also protected liver and kidney tissues against xylene produced cell damage.Conclusions:
The results of this study supports the view that xylene induces liver and kidney injury. Buffalo’s milk has potential to protect liver and kidney against xylene toxicityKeywords
1. Background
Xylene is widely used as a solvent in the printing, paint and rubber industries, it is also reported to be in cigarette smoke and is used in histological laboratory for tissue processing (1). Exposure to xylene can occur inhalation ingestion and/or skin contact.
Xylene is mainly metabolized in the liver and conjugated with glycine and excreted in the urine as methyl hippuric acid. It causes health effects and can injure the various organs including the liver and kidney. Symptoms like nausea, vomiting, poor appetite and gastric discomfort of GI tracts were reported in workers exposed to xylene vapors (1).
Ketan et al. found that the inhalation of xylene resulted in significant dose-dependent biochemical and functional changes in the mice liver and kidney (2). Liver injury was reported in workers occupationally exposed to xylene (3). The liver is the main organ responsible for the metabolism of xenobiotics and is also the primary organ for many organic solvents. In addition to the liver, xylene induces kidney injury (4).
The mechanism by which xylene induces liver and kidney injury is not completely understood. However, the large body of evidence indicated that this chemical manifest is caused by the toxicity by lipid peroxidation in the liver as well as in the kidney (5). Many recent studies showed that milk has an antioxidant capacity, represented by the natural occurring of vitamin E and C, beta-carotene and enzymatic systems (6). Arab et al. found that Camel’s milk ameliorates trinitrobenzene sulfonic acid (TNBS)- induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress (7). Protective activity of the camel’s milk against toxicity of paracetamol in rat liver was also reported (8). Khan found hepatoprotective effects of camel’s milk against CCl4-induced hepatotoxicity in rats (9).
Buffalo are the second most valuable species in the world for milk production. Buffalo’s milk is extremely rich in calcium and is a good source of minerals such as magnesium, potassium and phosphorus and is also a good source of vitamins and proteins (10). To our knowledge, the effect of BM on xenobiotic induced adverse effect on human and animals has not been reported. The aim of the present study was to evaluate the protective effects of buffalo’ milk against xylene-induced hepatotoxicity and nephrotoxicity in rats.
2. Methods
2.1. Animals and Treatment
Forty- two adult male Wistar rats (180-220 g) were maintained in a controlled environment (12 hour darkness-brightness and temperature 23±20) and fed on rodent chow and tap water ad libitum. Rats were divided randomly into seven groups (n=6/group). Rats in a group I were used as a control ( received vehicle only). Groups II, III and IV were pretreated with 1 mL distilled water (gavage) ten minutes prior to administration (ip) of xylene at doses of 0.81, 0.162 or 0.324 mmol/kg. Animals in a group of V, VI and VII were given 1 mL buffalo’s milk, BM (gavage) ten minutes prior to administration (ip) of xylene at doses of 0.81, 0.162 or 0.324 mmol/kg (11).
The experiment was repeated for 7 consecutive days. 24 hours after last experiment, all animals were killed from an over dose of sodium pentobarbital. The blood was collected for determination of biochemical parameters. Liver damage was estimated by measuring serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activity. Nephrotoxicity was evaluated by measuring blood urea nitrogen (BUN) and creatinine (CR) levels. In addition, superoxide dismutase (SOD), catalase (CAT) and gluthathione (GSH) were also determined. SOD, CAT activities and GSH levels were examined using a commercially available kit (Biovision) according to the manufacturer’s instructions.
The liver and kidney tissues were removed, fixed and processed for light microscopy. The tissues fixed in 10% buffered formalin for 24 hours, routinely processed and paraffin embedded. Five histological sections each at least 15 µm apart were taken from each tissue block and stained with Hematoxylin and Eosin, (H and E). This protocol was approved by the ethics committee of the Ahvaz Jundishapur University of Medical Sciences.
Biochemical data was expressed as mean ± standard error. The results were analyzed by analysis of variance, completely randomized design and treatment differences were identified by the method of Newman-Keuls. P < 0.05 was used as the criterion for significance. Six animals were used for each treatment group.
2.2. Buffalo’s Milk
Buffalo’s milk samples were collected daily, in the early morning, fromthe herd of buffalos by handmilking as normally practiced by the farmers. The samples were then collected and put in sterile screw bottles and kept in a cool box until being transported. The rats were given this fresh milk by oral delivery (gavage) 1 mL/animal as such without any further treatment.
3. Results
Treatment of male rats with xylene led to an increase in dose dependent manner the activities of serum enzymes level AST, ALT and ALP (Table 1).
| Parameters | Treatment | Does, mmol/kg | |||
|---|---|---|---|---|---|
| 0 | 0.081 | 0.162 | 0.324 | ||
| AST, u/dL | X | 23.59 ± 2.67 | 27.41 ± 3.32b | 31.23 ± 2.94b | 39.86 ± 3.56b |
| BM + X | 22.71 ± 1.67 | 21.24 ± 2.73 | 23.18 ± 1.85 | 25.72 ± 1.31 | |
| ALT, u/dL | X | 39.4 ± 4.97 | 42.84 ± 4.82 | 47.52 ± 3.72b | 50.36 ± 3.89b |
| BM + X | 38.3 ± 3.56 | 37.24 ± 2.39 | 38.15 ± 2.38 | 39.54 ± 2.89 | |
| ALP, u/dL | X | 99.31 ± 3.54 | 101.28 ± 2.14b | 112.62 ± 3.75b | 127.4 ± 3.67b |
| BM + X | 96.2 ± 4.2 | 98.8 ± 2.28 | 99.12 ± 3.75 | 100.33 ± 4.79 | |
Related increase in biochemical parameters was observed in xylene-treated when compared to those in control values. BM had no effect on blood biochemical parameters. However, pretreated of rats with BM markedly reduced all biochemical parameters in animals treated with various doses of xylene. The level of AST, ALT, and ALP significantly decreased in rats pretreated with BM and given various doses of xylene when compared to those that received vehicle and the same dose of xylene only (Table 1).
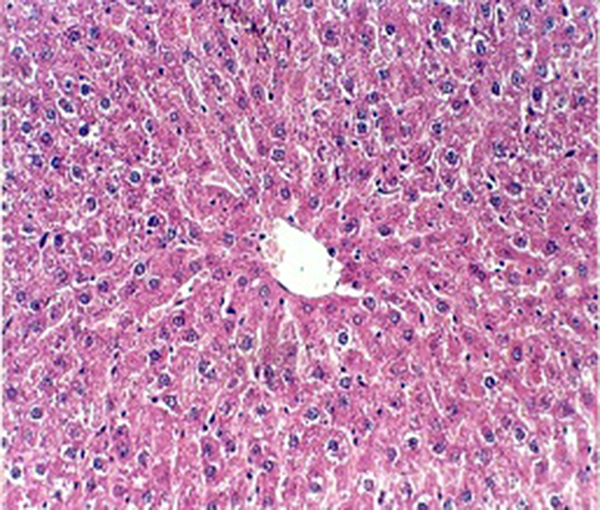
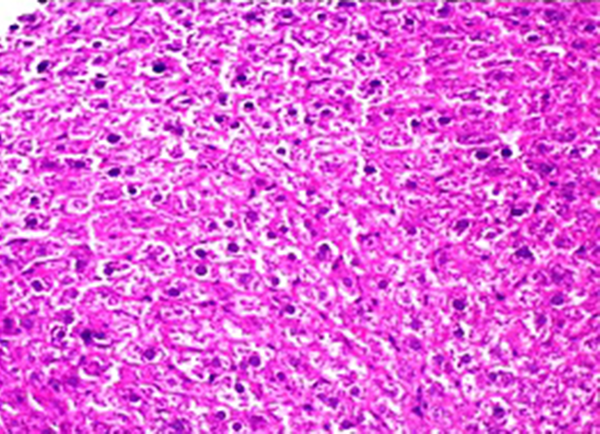
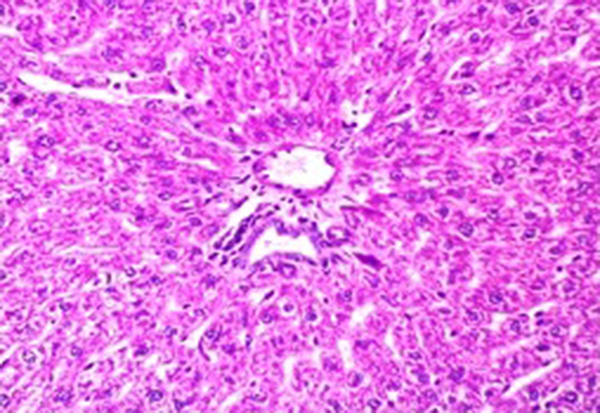
Administration of vehicle alone did not produce detectable injury in rat liver (Figure 1). In control rats (vehicle treated animals) liver cells were intact. There was no detectable injury in hepatocytes (Figure 1). However, there was a xylene-induced injury in the liver. The extant of injury appeared to be a dose related manner. However, the most remarkable histopathological alterations were noted in rats treated with 0.324 mmol/kg xylene. The liver cells were enlarged and granular with many vacuoles. The nuclei appeared to be dilated. Hydropic degeneration was obvious (Figure 2). Buffalo’s milk had no effect on the liver tissue and there was no obvious injury. However, this agent protected hepatocytes against xylene produced cytotoxicity (Figure 3).
Light Micrograph of Liver Control Rat Showing no Obvious Injury
Light Micrograph of Rat Liver Treated With 0.324 mmol/kg Xylene
Light Micrograph of Liver Rat Pretreated With Buffalo's Milk and Received 0.324 mmole/kg Xylene
A dose related increase in BUN and creatinine concentration was observed after treatment of animals with xylene when compared to those in control values. Elevation of renal biochemical parameters were predominant in 0.324 mmol/kg xylene treated rats (Table 2).
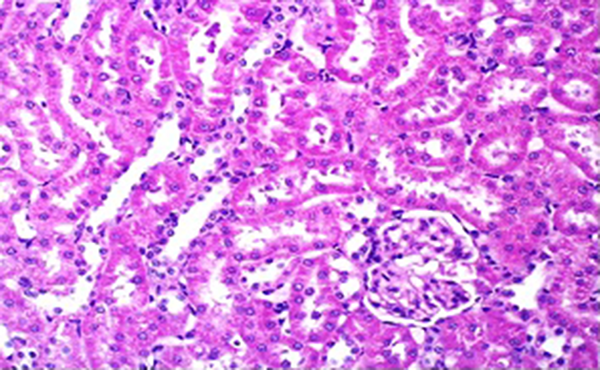
Buffalo’s milk had no effect on BUN and creatinine when compared to control animals. However, pretreated animals with this agent markedly reduced the level of biochemical parameters when compared to xylene treated rats (Table 2). Administration of vehicle alone did not produce detectable injury in rat kidney (Figure 4). However, dose-related injuries in xylene-treated rats were noted. Light microscopy revealed that renal tubular cells were swollen, had loss of staining capacity, nuclei appeared to be dilated and presence of blood clots were noted in xylene treated rats (Figure 5). Similarly, BM had no effect on kidney cells. However, the extant of xylene-induced injury decreased in BM pretreated rats when compared to those treated with xylene only (Figure 6).
Light Micrograph of Rat Kidney Treated With Vehicle (Control Rat) Showing the Normal Architecture of Renal Tissue
Light Micrograph of Rat Kidney Treated With 0.324 mmol/kg Xylene
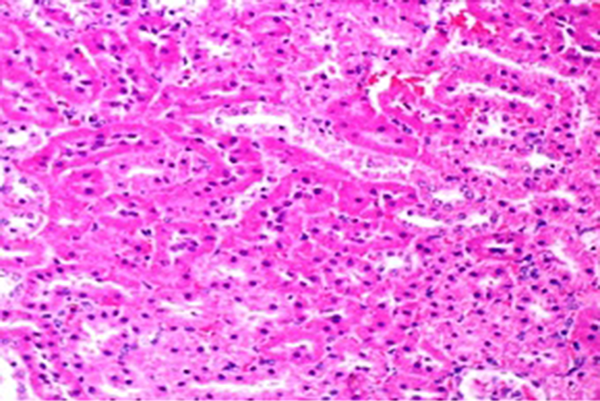
Light Microscopy of Kidney of Rats Pretreated With Buffalo's Milk and Subjected to 0.324 mmol/kg Xylene
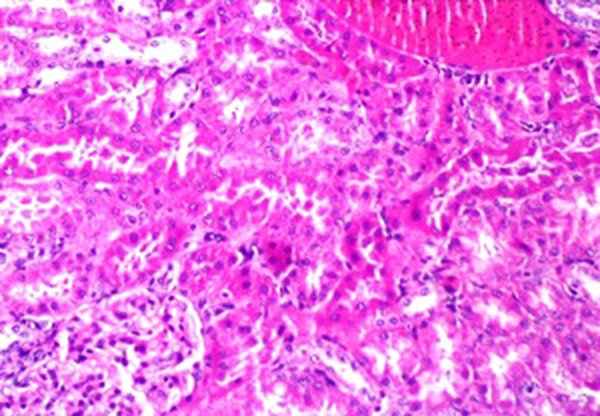
The levels of enzymatic (superoxide dismutase, SOD and catalase, CAT) and non-enzymatic glutathione (GSH) components of the antioxidant system were decreased in a dose dependent manner in xylene-treated rats. However, pretreated animals with Buffalo’s milk markedly increased the level of the antioxidant components in xylene treated rats (Table 3).
| Parameters | Treatment | Does, mmol/kg | |||
|---|---|---|---|---|---|
| 0 | 0.081 | 0.162 | 0.324 | ||
| SOD, u/mL | X | 22.79 ± 0.14 | 20.12 ± 0.32b | 15.32 ± 0.05b | 11.56 ± 0.23b |
| BM + X | 23.91 ± 0.67 | 22.6 ± 0.17 | 21.71 ± 0.85 | 18.78 ± 0.31 | |
| CAT, u/mL | X | 33.41 ± 4.97 | 31.4 ± 4.22 | 28.54 ± 3.72b | 24.63 ± 3.89b |
| BM + X | 34.93 ± 4.93 | 33.34 ± 4.39 | 32.9 ± 2.38 | 30.54 ± 2.89 | |
| GSH, mg/mL | X | 36.33 ± 2.54 | 33.18 ± 2.14b | 28.93 ± 3.75b | 19.34 ± 3.67b |
| BM + X | 37.42 ± 2.42 | 36.48 ± 2.28 | 35.23.2 ± 3.75 | 31.63 ± 2.79 | |
4. Discussion
Our data showed that xylene induced dose dependent injury in rat liver and kidney. Assessment of hepatoxicity is done by determination of liver enzymes including AST, ALT, ALP and histopathological observation. Nephrotoxicity was estimated by determination of BUN, creatinine (Cr) levels and histopathological examination. Elevation of liver enzymes as well as renal biochemical parameters (BUN and Cr) and histopathological damage in the liver and kidney were noted in a dose-dependent manner in rats exposed to xylene. Kum et al. showed that the exposure of xylene to a rat caused fat deposition and increase weight of the liver and kidney (12). Similarly, it was reported apoptosis and progressive renal fibrosis in the proximal renal tubules with long term exposure to xylene (12, 13).
We observed antioxidant enzymes including SOD and CAT and the level of GSH decreased in a dose-dependant manner in xylene treated rats. This finding suggests that generation of reactive toxic metabolites is responsible for xylene induced toxicity. Kum et al. reported that exposure to xylene induced oxidative stress and induced hepatotoxicity in female rats (13).
In this study, BM protected liver and kidney against xylene-induced toxicity. Al-Fartosi et al. found that camel’s milk protected liver cells against paracetamol caused hepatotoxicity in rats (8). Al-Hashem showed that camel’s milk protects aluminum chloride-induced toxicity in the liver and kidney of rats (14). It has also been reported the renoprotective potential of camel’s milk against cisplatin-induced oxidative stress and renal dysfunction in mice (15).
Vahdani manesh et al. found that the level of liver enzymes AST and ALT were increased in paint manufacturing industry workers, which had been chronically exposed to aromatic solvents including benzene, toluene and xylene. However, the levels of the enzymes reduced in workers after one year from drinking 200 mL milk daily. These authors reported that milk was effective in reducing aromatic solvent induced liver adverse effects in workers (16). We showed that BM increased the activity of SOD and CAT and the level of GSH in xylene treated rats. Our data demonstrated that BM protected rats against xylene-induced adverse effects in liver and kidney. This finding supports the view that the protective effects of BM could be contributed to its antioxidant activity.
4.1. Conclusion
Our biochemical and histopathological findings demonstrates that xylene-induced adverse effects on rat liver and kidney. Buffalo’s milk protects rats against xylene-induced toxicity. Therefore, buffalo’s milk could be beneficial for alleviating adverse effects of xylene.
Acknowledgements
References
-
1.
Langman JM. Xylene: its toxicity, measurement of exposure levels, absorption, metabolism and clearance. Pathology. 1994;26(3):301-9. [PubMed ID: 7991289].
-
2.
Ketan VK, Bhavyata K, Linzbuoy G, Hyacinth HN. Renal and hepatotoxic alterations in adult mice on inhalation of specific mixture of organic solvents. Toxicol Ind Health. 2015;31(12):1158-64. [PubMed ID: 23637306]. https://doi.org/10.1177/0748233713485892.
-
3.
Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol. 2012;18(22):2756-66. [PubMed ID: 22719183]. https://doi.org/10.3748/wjg.v18.i22.2756.
-
4.
Kum C, Sekkin S, Kiral F, Akar F. Effects of xylene and formaldehyde inhalations on renal oxidative stress and some serum biochemical parameters in rats. Toxicol Ind Health. 2007;23(2):115-20. [PubMed ID: 18203563].
-
5.
Rana SV, Kumar S. Lipid peroxidation in liver, kidney and brain of rats after combined exposure to xylene, toluene and methyl alcohol. Indian J Exp Biol. 1994;32(12):919-21. [PubMed ID: 7896327].
-
6.
Pihlanto A. Antioxidative peptides derived from milk proteins. Int Dairy J. 2006;16:1306-14.
-
7.
Arab HH, Salama SA, Eid AH, Omar HA, Arafa el SA, Maghrabi IA. Camel's milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem Toxicol. 2014;69:294-302. [PubMed ID: 24788059]. https://doi.org/10.1016/j.fct.2014.04.032.
-
8.
Al-Fartosi K, Khuon OS, Al-Tae HI. Protective role of camel's milk against paracetamol induced hepatotoxicity in male rats. Int J Res Pharmaceut Biomed Sci. 2011;2:1795-9.
-
9.
Khan AA, Alzohairy M. Hepatoprotective effects of camel milk against CCl4-induced hepatotoxicity in Rats. Asian J Biochem. 2011;6(2):171-80.
-
10.
Nasr MA. The impact of crossbreeding Egyptian and Italian buffalo on milk yield and composition under subtropical environmental conditions. J Dairy Res. 2016;83(2):196-201. [PubMed ID: 27210493]. https://doi.org/10.1017/S0022029916000194.
-
11.
Kaneko T, Wang PY, Tsukada H, Sato A. m-xylene toxicokinetics in phenobarbital-treated rats: comparison among inhalation exposure, oral administration, and intraperitoneal administration. Toxicol Appl Pharmacol. 1995;131(1):13-20. [PubMed ID: 7878667]. https://doi.org/10.1006/taap.1995.1041.
-
12.
Kum S, Sandiksi M, Eren U, Metin N. Effect of formaldehyde and xylene inhalation on fatty Liver and Kidney in adult and developing rats. J Anim Vet Adv. 2010;9(2):396-401.
-
13.
Kum C, Kiral F, Sekkin S, Seyrek K, Boyacioglu M. Effects of xylene and formaldehyde inhalations on oxidative stress in adult and developing rats livers. Exp Anim. 2007;56(1):35-42. [PubMed ID: 17283889].
-
14.
Al-Hashem F. Camel's milk Protects against aluminum chloride-induced toxicity in the liver and kidney of white albino rats. Am J Biochem Biotech. 2009;5(3):98-109.
-
15.
Afifi MEM. Effect of camel's milk on Cisplatin-induced nephrotoxicity in Swiss Albino mice. Am J Biochem Biotech. 2010;6(2):141-7.
-
16.
Vahdani manesh M, Abolhasannejad V, Moasheri N, Ketabi D. Effect of milk drinking on the level of liver enzymes among workers of paint manufacturing industry. Occup Med. 2012;3(3):39-45.