Abstract
Background:
Several studies showed that dopamine and norepinephrine improve retention and retrieval of memory. Methylphenidate is an enhancer of dopamine and norepinephrine in brain.Objectives:
In the present study, the effect of methylphenidate was evaluated on retention and retrieval of memory in young and aged mice using passive avoidance apparatus.Materials and Methods:
Animals were divided into groups (n = 8) as follows: test groups received electric shock plus methylphenidate (2.5, 5 and 10mg kg-1, i. P.), control group received electric shock plus normal saline and blank group received only electric shock. In all groups, step-down latency for both retention and retrieval test of memory was measured. Methylphenidate was administered immediately after receiving electric shock in the retention test, but methylphenidate was administered 23.5 hours after receiving electric shock in the retrieval test.Results:
The mean of step-down latency on day 4 was significantly higher compared to day 2 (P < 0.05) in all young and aged groups of mice. The best response was attained with 5 mg/kg of methylphenidate. In memory retention test, the mean of step-down latency in young groups that received 2.5 and 5 mg/kg methylphenidate was significantly longer(P < 0.05) than aged groups. However, this difference was not significant in memory retrieval test.Conclusions:
Methylphenidate may improve memory retention and retrieval.Keywords
1. Background
Memory is one of the most important functions of the brain. Memory is the process in which information is encoded, stored and retrieved (1). Certain neurotransmitters such as acetylcholine, noradrenaline, dopamine and serotonin are involved in memory formation (2-4). Methylphenidate is a stimulant drug related to amphetamine, which acts to increase the synaptic concentration of dopamine and noradrenaline by blocking their re-uptakes (5, 6). Methylphenidate has been used to treat attention-deficit/hyperactivity disorder (ADHD) in children. It is also used in depression, narcolepsy, brain injury, cancer, pain, cognitive disorders and immune deficiency (7, 8). Methylphenidate is abused to enhance cognitive abilities by different groups of people. Some studies indicated that methylphenidate has cognitive enhancing properties, while others contradict this. For example, improvement in spatial working memory in healthy adults was reported by Mehta (9). Similar findings concerning spatial working memory improvement have been found by Elliott et al. (10). Schermer et al. reported that methylphenidate has no effect on concentration or sustained attention in healthy volunteers (11). Turner et al. reported no improvements in spatial span and spatial working, response inhibition (stop-signal) or sustained attention (rapid visual information processing) in their study population (12).
2. Objectives
The aim of this research was to investigate the effect of methylphenidate on retention and retrieval of memory in young and aged mice.
3. Materials and Methods
3.1. Animals
Young (aged 3 months) and old (aged 15 months) male Wistar albino mice were used during the study. The animals were purchased from the animal house of Jundishapur University of Medical Sciences, Ahvaz, Iran. They were kept in a clean holding room on a 12-hour light and dark cycle with relative humidity of 45-55% and temperature of 23 ± 2°C. During the experimentation, all mice were fed with concentrated food pellets (Pars Khurakdam Shushtar, Iran) and tap water ad libitum (13).
3.2. The Experiments
In this study, two groups of young adult and aged mice were used. Each group divided into five sub groups (n = 8) subsequently. The test groups received methylphenidate 2.5, 5 and 10 mg/kg. The Control group received normal saline (1 mL/100g) and the blank group was untreated. The step-down apparatus used to test passive avoidance, consisted of a box 25 × 25 × 20 cm in diameter with an electrifiable grid floor. There was a round plastic which could be enclosed by a 20-cm long hollow plastic cylinder with an inner diameter of 10 cm. On the first day, groups of four animals were given access to learning apparatus for three minutes to be familiarized with the new environment. On the second day, mice were individually placed on the platform inside the cylinder and after 10 seconds the cylinder was removed and the step-down latency was measured. Animals with latencies longer than 30 seconds were excluded from the study. On the third day, the same procedure was followed as the second day, except that a one-second foot shock (1 mA) was administered as soon as the animals left the platform with all four legs. Drugs were injected to animals immediately after foot shock, to study the effects on retention of memory. After 23.5 hours of shock, the same drug was injected to study the effect of retrieval of memory. On the fourth day, step-down latency of the mice was recorded. Each animal was used only once. All drugs were administered intraperitoneally (13).
3.3. Statistical Analysis
Results were expressed as means ± SEM. The data was analyzed using Student T-test and One-way ANOVA followed by LSD test. P < 0.05 was considered statistically significant.
4. Results
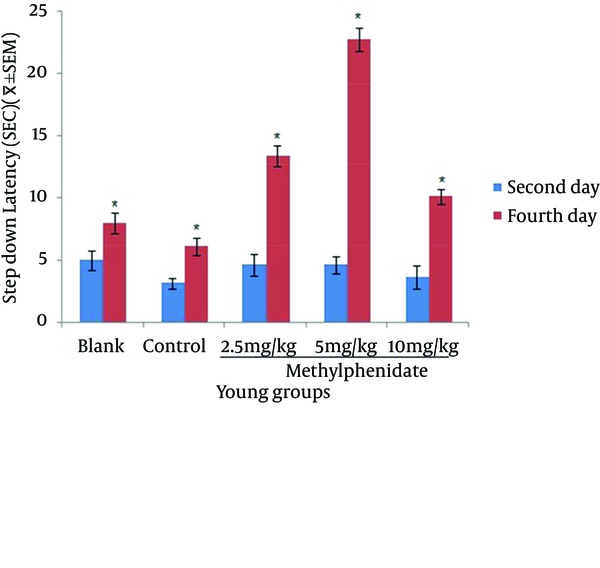
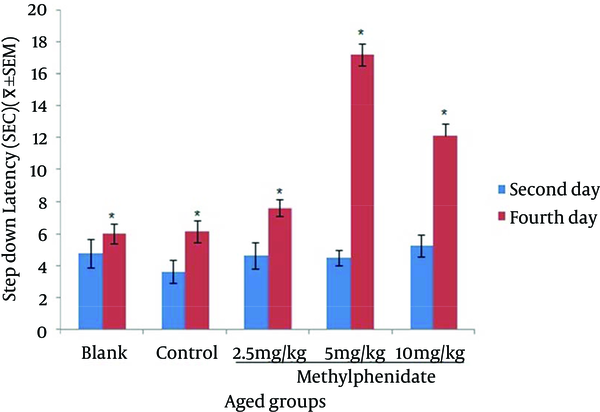
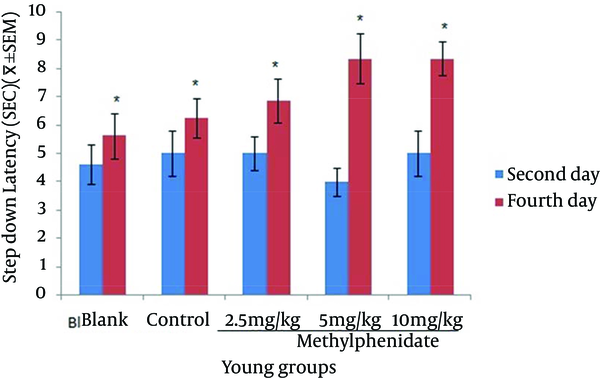
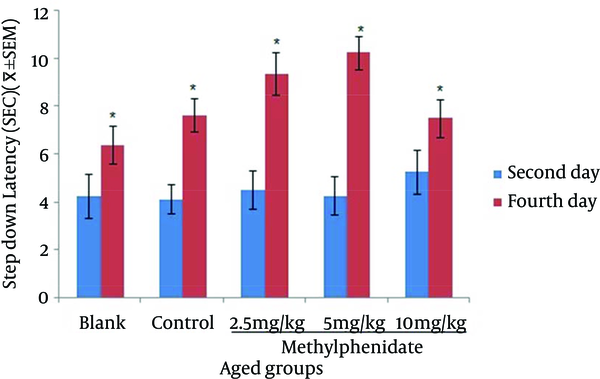
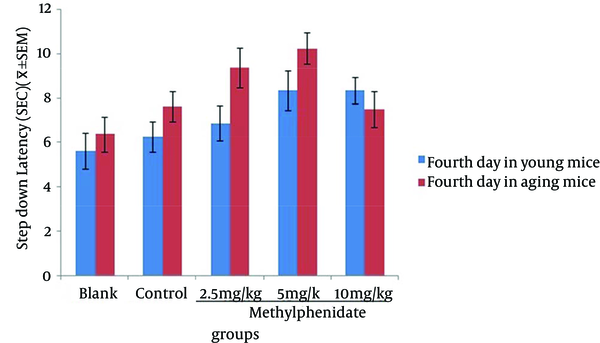
The mean of step-down latency on day four was higher compared to day two (P < 0.05) in retention and retrieval of memory in all young and aged groups of mice (Figures 1-4). There was a significant increase (P < 0.05) in the mean of step-down latency on day four regarding retention of memory in young mice group,which received 5 mg/kg methylphenidate compared to other groups. In addition, there was a significant increase (P < 0.05) in the mean of step-down latency of group that received 2.5 mg/kg methylphenidate compared to control group (Figure 5). In memory retention test, the mean of step-down latency on day four of aged mice group that received 10 mg/kg methylphenidate,was higher (P < 0.05) than other groups (Figure 6). Comparing the mean of step-down latency on day four showed no significant difference in retrieval of memory in all young and aged mice groups (Figures 7and 8). In memory retention test, the mean of step-down latency on day four of young mice groups that received 2.5 and 5 mg/kg was higher (P < 0.05) than aged mice groups, the difference was not significant in memory retrieval test (Figures 9 and 10).
Comparison of the Step-down Latency in Young Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retention Test in the Second and Fourth Days of Study.
Comparison of the Step-down Latency in Aged Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retention Test in the Second and Fourth Days of Study.
Comparison of the Step-down Latency in Young Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory RetrievalTest in the Second and Fourth Days of Study.
Comparison of the Step-down Latency in Aged Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retrieval Test in the Second and Fourth Days of Study.
Comparison of the Step-down Latency in Young Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retention Test in Fourth Day of Study.
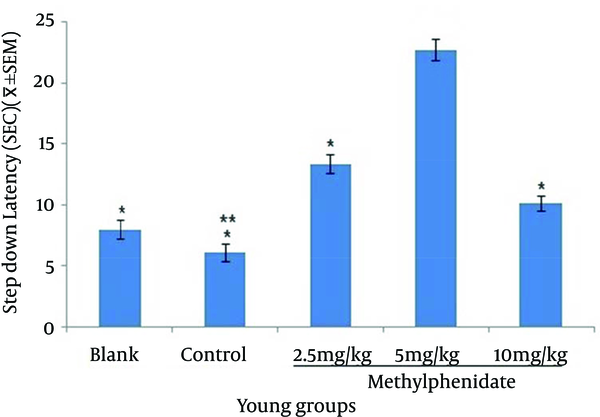
Comparison of the Step-down Latency in Aged Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidatein Memory Retention Test in Fourth Day of Study.
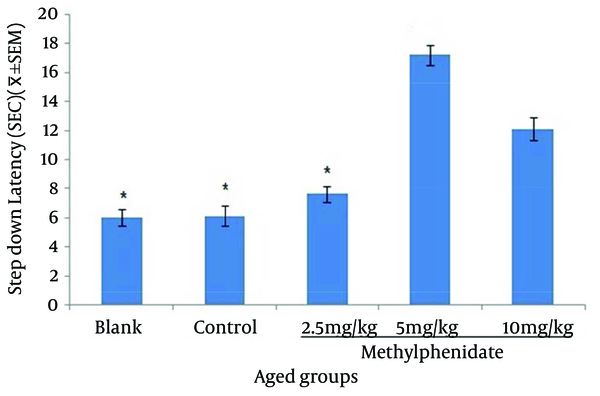
Comparison of the Step-down Latency in Young Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retrieval Test in Fourth Day of Study.
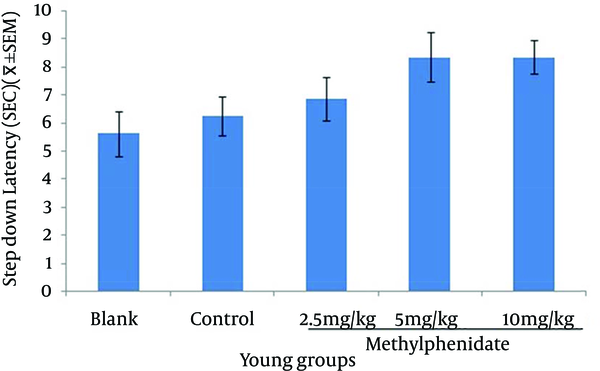
Comparison of the Step-down Latency in Aged Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retrieval Testin Fourth Day of Study.
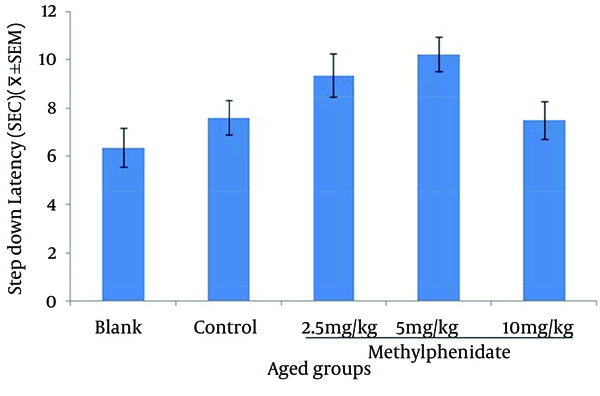
Comparison of the Step-down Latency in Young and Aged Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retention Test in Fourth Day of Study.
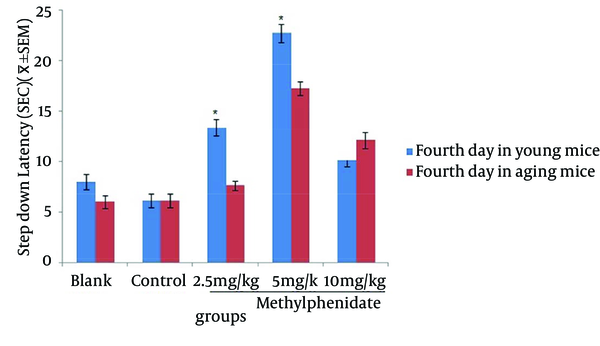
Comparison of the Step-down Latency in Young and Aged Mice (n = 8) That Received (Blank) No Injection, (Control) Normal Saline (10 mL/kg), (Test) 2.5, 5 and 10 mg/kg Methylphenidate in Memory Retrieval Test in Fourth Day of Study.
5. Discussion
Different nervous systems including cholinergic, dopaminergic, adrenergic, serotonergic and gabaergic are able to moderate memory function (14, 15). Studies have shown that inhibition of neurotransmitter in cholinergic system and stimulation of serotonergic system may impairmemory and learning. On the other hand, stimulation of dopaminergic and adrenergic systems improve memory and learning (2-4). Based on the mentioned documents, the effect of methylphenidate (indirect catecholamine agonists) on memory retention and retrieval was studied. The results showed that all doses of methylphenidate (2.5, 5, 10 mg/kg) improved memory retention and retrieval in young and old mice. The best effect on memory retention was observed at the dose of 5 mg/kg. The results of this study showed that methylphenidate improved memory retention in young mice significantly at doses of 2.5 and 5 mg/kg, but not in old mice. No significant difference was detected between young and old mice on memory retrieval. Other studies showed that α-2 adrenoceptor agonists improved memory in neurological disorders such as Alzheimer disease (16). In another study,Yonkov et al. showed that stimulation of the dopaminergic and adrenergic systems by intraperitoneal injection of strychnine (20 mg/kg) and amphetamine 1 mg/kg enhanced the memory retention (17). Such finding is consistent with the results of the present study. In a study conducted by Lazarova-Bakarova et al. adrenergic system stimulation by clonidine increased memory retention, which confirms the findings of our study (18). In a study, 5 mg/kg dosage of norepinephrine enhanced memory, whereas high doses had no effect on memory. Norepinephrine may improve memory process through some indirectmechanisms, such as change in heart-vascular amends, because it does not appear to cross the blood-brain barrier (19). It has been shown that stimulation of the central dopaminergic system improves memory and learning, so that injecting dopaminergic agonists such as apomorphine and ergotamine into the hippocampus improves memory retrieval, which is consistent with our results (4). High doses of apomorphine and bromocriptine as dopaminergic receptor agonists reduce the memory retrieval. High doses of sulpiride diminish memory retrieval by blocking Pre-synaptic D2 receptor (20). The above results confirm our finding about memory retrieval.In conclusion, methylphenidate may improve memory retention and retrieval probably due to releasing norepinephrine and dopamine in CNS and preventing re-uptake of these neurotransmitters. However, more studies are required to ascertainthe exact role of methylphenidate in retention and retrieval of memory.
Acknowledgements
References
-
1.
Alikatte KL, Akondi BR, Yerragunta VG, Veerareddy PR, Palle S. Antiamnesic activity of Syzygium cumini against scopolamine induced spatial memory impairments in rats. Brain Dev. 2012;34(10):844-51. [PubMed ID: 22475379]. https://doi.org/10.1016/j.braindev.2012.02.008.
-
2.
Essman WB. Age dependent effects of 5-hydroxytryptamine upon memory consolidation and cerebral protein synthesis. Pharmacol Biochem Behav. 1973;1(1):7-14. [PubMed ID: 4775586].
-
3.
Jaffard R, Destrade C, Durkin T, Ebel A. Memory formation as related to genotypic or experimental variations of hippocampal cholinergic activity in mice. Physiol Behav. 1979;22(6):1093-6. [PubMed ID: 493385].
-
4.
Grecksch G, Matties H. The role of dopaminergic mechanisms in the rat hippocampus for the consolidation in a brightness discrimination. Psychopharmacology (Berl). 1981;75(2):165-8. [PubMed ID: 6798605].
-
5.
Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3(5):386-96. [PubMed ID: 9774771].
-
6.
Scheel KJ, Braestrup C, Nielson M, Golembiowska K, Mogilnicka E. Cocaine: Discussion on the Role of Dopamine in the Biochemical Mechanism of Action. 1977;21:373-407. https://doi.org/10.1007/978-1-4684-3087-5_19.
-
7.
Leonard BE, McCartan D, White J, King DJ. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum Psychopharmacol. 2004;19(3):151-80. [PubMed ID: 15079851]. https://doi.org/10.1002/hup.579.
-
8.
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121. [PubMed ID: 11160455].
-
9.
Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20(6):RC65. [PubMed ID: 10704519].
-
10.
Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl). 1997;131(2):196-206. [PubMed ID: 9201809].
-
11.
Schermer M, Bolt I, de Jongh R, Olivier B. The Future of Psychopharmacological Enhancements: Expectations and Policies. Neuroethics. 2009;2(2):75-87. https://doi.org/10.1007/s12152-009-9032-1.
-
12.
Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Relative lack of cognitive effects of methylphenidate in elderly male volunteers. Psychopharmacology (Berl). 2003;168(4):455-64. [PubMed ID: 12734634]. https://doi.org/10.1007/s00213-003-1457-3.
-
13.
Saha N, Datta H, Sharma PL. Effects of morphine on memory: interactions with naloxone, propranolol and haloperidol. Pharmacology. 1991;42(1):10-4. [PubMed ID: 2057517].
-
14.
Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl). 1990;101(1):27-33. [PubMed ID: 2343073].
-
15.
Sudha S, Lakshmana MK, Pradhan N. Chronic phenytoin induced impairment of learning and memory with associated changes in brain acetylcholine esterase activity and monoamine levels. Pharmacol Biochem Behav. 1995;52(1):119-24. [PubMed ID: 7501653].
-
16.
Avery R. The Alpha-2A-Adrenoceptor Agonist, Guanfacine, Increases Regional Cerebral Blood Flow in Dorsolateral Prefrontal Cortex of Monkeys Performing a Spatial Working Memory Task. Neuropsychopharmacology. 2000;23(3):240-9. https://doi.org/10.1016/s0893-133x(00)00111-1.
-
17.
Yonkov DI. Possible role of brain dopaminergic systems in the memory effects of central stimulants. Methods Find Exp Clin Pharmacol. 1984;6(5):235-9. [PubMed ID: 6147449].
-
18.
Lazarova-Bakarova MB, Petkova BP, Todorov IK, Petkov VD. Memory impairment induced by combined disturbance of noradrenergic and dopaminergic neurotransmissions: effects of nootropic drugs. Acta Physiol Pharmacol Bulg. 1991;17(1):29-34. [PubMed ID: 1667717].
-
19.
Arzi A, Rahmat H. The effect of Phenytoin on retention and retrieval of memory in mice. J Babol Univ Med Sci. 2005;7(3):34-9.
-
20.
Zarrindast MR, Sadegh M, Shafaghi B. Effects of nicotine on memory retrieval in mice. Eur J Pharmacol. 1996;295(1):1-6. [PubMed ID: 8925865].