Abstract
Background:
Zero-valent iron (ZVI) can effectively activate persulfate (PS) generating free sulfate radicals (SO4•–), thereby presenting a promising technology to degrade recalcitrant organic contaminants such as para-chlorophenol (PCP) in wastewater.Objectives:
The current study aimed to examine the feasibility and application of ZVI/PS system through batch experiments to degrade PCP of petrochemical effluent, which its treatment is included in The United States environmental protection agency (USEPA) priority pollutant list.Materials and Methods:
Effects of dosages of ZVI (0.056 - 2.8 g/L), ZVI to PS molar ratio (0.1 - 5.0), PS concentration (2.5 - 25.0 mM/L), pH = (3.0 - 11.0), contact time (5 - 240 minutes), and ZVI reusability (three cycles) on PCP degradation were examined.Results:
The results showed that the PCP degradation increased with an increase in ZVI dosage from 0.056 to 1.4 g/L, an increase in persulfate concentration from 2.5 to 15.0 mM/L, and an increase in ZVI to PS molar ratio from 0.1 to 2.5. The optimal initial pH for PCP removal was 5.0 and the maximum removal efficiency of 70% was achieved within 120 minutes. Moreover, the ZVI catalyst was reused until the third cycle to activate the persulfate and degrade PCP. However, the degradation efficiency of PCP gradually decreased to 51.7% when the ZVI reuse time increased.Conclusions:
The results indicate that using ZVI/PS system is not an efficient enough method to treat petrochemical effluent, due to the complexity of petrochemical wastewater matrix and high- total dissolved solids (TDS) content, as well.Keywords
Persulfate Zero-Valent Iron Para-Chlorophenol Petrochemical Effluent
1. Background
Increased generation of hazardous chemicals and their subsequent release have resulted in severe environmental pollution. Chlorophenols are a large group of chemicals with chlorine atoms (between one and five) attached to the phenolic structure, and totally 19 congeners exist (1). Chlorophenols (CPs) present a sizable fraction of overall organic chemical compounds annually produced and consumed by a number of industries, such as pesticides, pulp and paper, antiseptics, pharmaceuticals and dyes (2-4). As a result they can be frequently found in surface and ground waters, soil and industrial wastewaters. They are considered harmful for human health due to their potential carcinogenic and mutagenic activity and toxicity and therefore, are listed among the priority contaminants of major environmental concern (5-7). According to Sze and McKay (1), the world market of chlorophenols is fairly stable and is ca. 100 kilotons per year, in which the production of heavy and light chlorophenols is ca. 25 - 30 and 60 kilotons per year respectively. Therefore, the removal of these kinds of pollutants is of great interest, since conventional biological treatment technologies are not able to do it efficiently. Advanced oxidation processes (AOPs) are quite appropriate to remediate wastewater as a pre or post treatment process. AOPs which typically involve strategies to generate hydroxyl radicals (OH), due to high redox potential (1.9 - 2.7 V), efficiently degrade such pollutants (1, 8, 9). Examples of such AOPs include UV/H2O2, vacuum UV, O3/H2O2, electrochemical oxidation and ultrasonic irradiation, all of which degrade CPs effectively (3, 10-13).
There has been recent interest to generate sulfate radical (SO4•–) in AOPs due to its high redox potential of (2.5 - 3.1 V), which can oxidize most of the organics in water (14-16). Besides the high redox potential of PS can be applied in chemical oxidation due to its high solubility in water (2.5 M at 20°C), high stability and relative low cost. PS can be thermally or chemically activated, either by pH adjustment, or employing transition metals (17, 18). However, (SO4•–) is more selective than (OH) while still reacting rapidly with many organic substrates (15) (SO4•–) can be generated by activation of proxy disulfate or proxy monosulfate using UV, heat, base, or transition metals (8, 19-21).
Although the aforementioned studies illustrate the effectiveness and potential of persulfate for environmental applications, in situ activation of persulfate by heat or UV can be challenging, especially in an industrial wastewater scenario.
Numerous studies demonstrated that (SO4•–) can degrade endocrine disrupting compounds, chlorinated compounds, pesticides, dyes, methyl tert butyl ether, drugs, aniline, p-nitro phenol, per fluorinated compounds and 2-methylisoborneol (MIB) and geosmin (14, 15, 19, 22-24). In recent years, the oxidation of different environmental contaminants by persulfate activated by zero-valent iron (Fe°) is investigated (17, 25, 26). Fe° could serve as a slow-releasing source of dissolved Fe2+ which would activate persulfate to produce sulfate radical, as described in the following Equations 1 and 2:
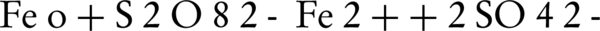
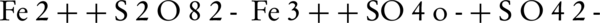
Rodriguez et al. (17) reported that the zero-valent iron (ZVI) could be used as a releasing source of ferrous iron through corrosion by PS (Equation 1). In addition to this oxidation reaction, two other oxidation pathways may take place; one depending on the oxidation of ZVI by ferric ion, and the other mediated by the H+ corrosion of ZVI Equations 3 and 4 (17).
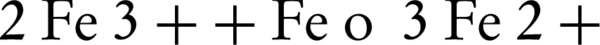
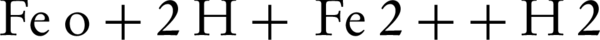
2. Objectives
The current study aimed to evaluate Fe° as an alternative catalyst to activate PS and treat petrochemical wastewater containing PCP. The performance of the Fe°/PS process to improve the degradation of PCP was evaluated through the examination of various operational parameters, including Fe° dosage, initial solution pH and initial concentration of PS. Fe° reusability was also evaluated.
3. Materials and Methods
3.1. Materials
Analytical reagent grade or better chemicals as well as deionized water were used in the experiments. Para-chlorophenol (PCP ≥ 99%) was purchased from Sigma-Aldrich. Sodium persulfate (PS ≥ 98%) was obtained from Sigma-Aldrich and its stock solution was prepared in deionized water. Zero Valent Iron (ZVI or Fe° ≥ 99.9% and particle diameter < 10 µm) from Aldrich were used as the PS activator. All the stock solutions used in this study were stored in a refrigerator at 4°C in the dark. All other chemicals were of analytical grade and were used without further purification.
3.2. Wastewater
The petrochemical high-total dissolved solids (TDS) wastewater was collected from effluent of high-TDS wastewater treatment plant of a petrochemical company in Bandar-e-Imam Petrochemical Complex, Iran. The initial characterization showed that the wastewater had a COD of 750 ± 65 mg/L, TDS of 17420 ± 3500 mg/L and a pH of 7.35 ± 0.25. This wastewater was stored was frozen deeply and used for further studies.
3.3. Batch oxidation experiments
Stock solutions of PCP (50 mg/L) and persulfate (10 g/L, or 52.08 mM) were prepared in petrochemical wastewater and deionized water, respectively, prior to each batch experiment. Fifty milliliters of the PCP containing wastewater and 0.5 mL of the persulfate stock solution and then appropriate dosages of ZVI were added simultaneously to a 150 mL bottles, shaken at 150 rpm in an orbital shaker at 25 ± 1°C, to give initial concentrations of PCP and persulfate of 50 mg/L and 10 mM/L, respectively. To examine the effects of ZVI on PCP oxidation by persulfate, a pre-determined amount of ZVI was added to the PCP-persulfate solution. The following ZVI doses were examined at a fixed amount of persulfate: 0, 0.056, 0.14, 0.28, 0.56, 1.4 and 2.8 g/L ZVI (each containing 10 mM/L of PS).
For the pH effect experiment, the oxidation of PCP by ZVI/PS was investigated at the pH range of 3 - 11. The initial pH of the PCP containing wastewater was adjusted by 1.0 M/L H2SO4 and 1.0 M/L NaOH and pH was detected using a pH meter (Met Rohm model 713 pH meters). Except for the pH effect experiment and the reuse study of ZVI, the pH of all the original wastewater was 7.35 ± 0.25. At selected time intervals (0, 5, 10, 15, 30, 60, 120, and 240 minutes), a 2 mL sample was collected from each replicate bottle and immediately passed through a 0.22 μm membrane filter (Millipore, MA, USA) for PCP analysis. After filtration, methanol (1.0 mL of methanol for each 2 mL sample), a well-known quenching agent for sulfate radicals, was added and vigorously shaken for five minutes using a vortex shaker to stop the reaction before chemical analysis. For each experiment, controls without ZVI or persulfate were run in parallel under identical conditions.
During the reuse study of ZVI, appropriate doses of ZVI were set into the 150 mL bottles containing PCP solutions (50 mL, 50 mg L-1). Then they were shaken vigorously for approximately two hours. In the next step, the ZVI was separated magnetically and cleansed sequentially via both distilled and deionized water several times and used in three sequential cycles.
All the experiments were prepared in triplicates to ensure reproducibility and to estimate experimental errors. Oxidation efficiency of PCP (R) was calculated according to the following Equation 5:
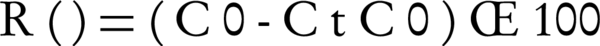
Where R (%) represented the PCP removal efficiency, C0 (mg L-1) was the initial concentration of PCP in the solution, and Ct (mg L-1) stood for the concentration of PCP at t minute.
3.4. Analytical Methods
The concentration of PCP was measured using direct photometric method, according to the procedure of the standard methods to examine water and wastewater (5530 D) by 4-amino anti pyrine and potassium ferric cyanide reagents as well as UV-vis spectro photometer (DR 5000, HACH) by measuring the absorption at a wave length of 500 nm (27).
4. Results
4.1. Effect of Fe° Dosage and the Molar Ratio of Fe° to PS on PCP Oxidation
To investigate the effect of Fe° dosage on the degradation of PCP, a series of comparative experiments were conducted by adding different amounts of Fe°, while a fixed initial concentration of persulfate (10 mM) was used with the natural pH of petrochemical high-TDS wastewater (7.35 ± 0.25). As shown in Figure 1, the PCP degradation rate was heavily influenced by the Fe° dosage and the molar ratio of Fe° to PS at a fixed PS concentration. The removal percent of PCP was increased from 8.2% to 47.0% when the Fe° dosage went up from 0.056 g/L (molar ratio of [ZVI]: [PS] = 0.1) to 0.56 g/L (molar ratio of [ZVI]: [PS] = 1.0), then it remained unchanged when the Fe° dosage increased from 0.56 g/L (molar ratio of [ZVI]: [PS] = 1.0) to 1.4 g/L (molar ratio of [ZVI]: [PS] = 2.5), but gradually decreased to 37.3% when the Fe° dosage further increased to 2.8 g/L (molar ratio of [ZVI]: [PS] = 5.0). Too high Fe° dosage likely provided too much Fe2+ that might scavenge (SO4•–) produced in the ZVI/PS system (Equation 6), thereby reducing the overall degradation rate and efficiency.
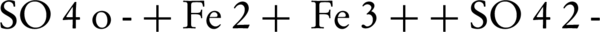
Effect of Initial Fe° Dosage on PCP Degradation of Petrochemical High-TDS Wastewater in the ZVI/PS System
![Effect of Initial Fe° Dosage on PCP Degradation of Petrochemical High-TDS Wastewater in the ZVI/PS System Experimental conditions: [PCP] = 50 mg/L; [PS] = 10.0 mM; room temperature (25 ± 1°C); reaction time 240 minutes, no pH adjustment (7.35 ± 0.25).](http://services.brieflands.com/cdn/serve/313ea/5c4d9961fa6dc75c1c8ca8c10c4a7cdea6dbe960/jjhs-8-2-35108-i001-preview.png)
It is reported that Fe2+ is one of the strongest species that can catalyze PS to generate (SO4•–) which can significantly improve the efficiency of the pollutant degradation (22, 28). The previous studies showed that Fe° can be applied as the source of Fe2+ in acid media in Fenton reactions (29). In the current study, the combination of Fe° and PS was also highly effective for PCP degradation in petrochemical high-TDS wastewater. With the increase of the Fe° dosage, the amount of (SO4•–) in ZVI/PS system increased, consequently leading to the increase of the PCP degradation efficiency. However, too high Fe° dosage could not achieve the high PCP degradation efficiency, since the presence of excessive Fe2+ rapidly activated all the PSs and then acted as the scavenger of (SO4•–), accounting for the decrease in the oxidation of PCP.
Therefore, the optimum Fe° dosage and Fe° to PS molar ratio was 0.56 - 1.4 g/L and 1.0 - 2.5, respectively, in the current study. The findings were consistent with those of the previous studies that the optimal Fe° to PS molar ratio was 1: 1, and complete oxidation of polyvinyl alcohol (PVA) (30) and 93.2% oxidation of acetaminophen (28) were obtained by Fe° activated PS in 120 minutes. The optimum Fe° dosage of 1.0 g/L would be used in the following experiments.
4.2. Effect of Initial Per-Sulfate Concentration
The effect of initial PS concentration on PCP degradation was investigated with six S2O82- concentrations (2.5, 5.0, 10.0, 15.0, 20.0 and 25.0 mM) at pH 7.35 ± 0.25 and 25°C with a fixed ZVI dosage (1.0 g/L). The results were displayed in Figure 2. The degradation efficiency of PCP increased from 13.7% to 57.7% when the concentration of PS rose from 2.5 mM to 15.0 mM. However, it was observed that further increase in PS concentration resulted in a decline in PCP degradation. These were mainly due to the PS depletion when the initial PS concentration was insufficient. By increasing the concentration of PS, more PS molecules could reach the ZVI surface and react with Fe°, which resulted in improved PCP degradation. Nevertheless, since the ZVI dosage was fixed, the available amount of (SO4•–) was limited. Therefore when excess PS participated in reacting with ZVI to degrade PCP, the PS competed with PCP for sulfate radicals, which resulted in decreased degradation efficiency (Equation 7). On the other hand, the scavenging effect of (SO4•–) (Equation 8) reduces degradation efficiency of PCP in ZVI/PS system.
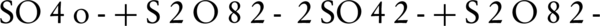

This result is similar to the observations of persulfate/Fe2+ degrading azo dye orange G at 30 mg/L (31) and p-nitro aniline removal from wastewater by persulfate/Fe3O4 nanoparticles process at 0.2 mM/L (16).
Effect of Initial PS Concentration on PCP Degradation of Petrochemical High-TDS Wastewater in the ZVI/PS System
![Effect of Initial PS Concentration on PCP Degradation of Petrochemical High-TDS Wastewater in the ZVI/PS System Experimental conditions: [PCP] = 50 mg/L; [ZVI] = 1.0 g/L; room temperature (25 ± 1°C); no pH adjustment (7.35 ± 0.25).](http://services.brieflands.com/cdn/serve/313ea/4443d82688a558caf1bd6ff6a4cd39ce1d97e61f/jjhs-8-2-35108-i002-preview.png)
4.3. Effect of Initial pH
The pH value of the reaction solution is usually a crucial parameter affecting the oxidative degradation of organic pollutants. The pH value strongly influences the dissolution of Fe2+ from ZVI, which affects the degradation efficiency of PCP. Therefore, the influence of initial pH on the degradation efficiency of PCP was studied through different pH values ranging from 3.0 to 11.0. All of the experiments were conducted at 25°C, 15.0 mM S2O82, 1.0 g/L ZVI and 50 mg/L PCP.
The PCP degradation under different initial pH values are shown in Figure 3. It can be observed that PCPoxidation by persulfate activated with ZVI was a pH-dependent process, where the degradation efficiency generally decreased from low to high initial pH. Compared with alkaline solution, rapid degradation of PCP was observed under the acidic condition. After 120 minutes, about 70% of PCP was removed at pH = 5.0; then it remained almost unchanged when the pH decreased from 5.0 to 3.0, whereas only 20.5% was transformed at pH = 11.0. Generally, an acidic condition facilitates the formation of Fe2+ from the ZVI surface (Equation 9) that generates more (SO4•–), in the ZVI/PS system.
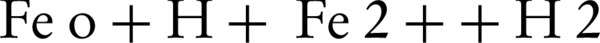
However, the precipitation of Fe3+ occurred when the pH was above 5.0, leading to the adsorption of iron hydroxides onto the surface of ZVI, and consequently formed electronic barriers, which inhibited the corrosion of ZVI and release of Fe2+. These results were similar to those of the degradation of acetaminophen in water by Fe°/PS system (28), lindane in aqueous solution by Fe2+-activated sodium persulfate (22) and p-nitro aniline in wastewater by persulfate/Fe3O4 (16). Hence, the pH to degrade PCP in ZVI/PS system was 5.0.
Effect of Initial pH on PCP Degradation of Petrochemical Hig-TDS Wastewater in The ZVI/PS System
![Effect of Initial pH on PCP Degradation of Petrochemical Hig-TDS Wastewater in The ZVI/PS System Experimental conditions: [PCP] = 50 mg/L; [ZVI] = 1.0 g/L; [PS] = 15.0 mM; room temperature: 25 ± 1°C.](http://services.brieflands.com/cdn/serve/313ea/c2eb29d04f2a528401a74117d370e40ed28eeda7/jjhs-8-2-35108-i003-preview.png)
4.4. The Study of Recycling and Reusing of ZVI
It is difficult to collect the excess amounts of ferrous catalyst after its addition to the wastewater, and results in a substantial waste of resources. However, magnetic materials such as iron (Fe°), can be used to address this dilemma. They can be easily removed from the reactor at the end of each repetitive oxidation process, and then used in succeeding runs. To evaluate the reusability of the catalyst, ZVI was repeatedly used to activate the persulfate three times. After each run, the catalysts were collected using a magnet, rinsed with distilled water several times, and then recycled for the next batch. Figure 4 show that when the ZVI cycle time increased, the degradation efficiency of PCP gradually decreased from 72.4% to 51.7%. The ZVI was used until the third cycle to activate the persulfate and degrade PCP; however, the removal efficiency was only 51.7%. This result demonstrated that the activating ability of the ZVI catalyst after being used four times was negligible on the account that the degradation efficiency can reach 51.7% only with persulfate in the reaction. The decrease in the removal efficiency can be explained by the conversion of the Fe2+ to Fe 3+ on the surface of ZVI.
The PCP Degradation in Multi-Cycle Batch Experiments in the ZVI/PS System
![The PCP Degradation in Multi-Cycle Batch Experiments in the ZVI/PS System Experimental conditions: [PCP] = 50 mg/L; [ZVI] = 1.0 g/L; [PS] = 15.0 mM; initial pH = 5.0; room temperature: 25 ± 1°C.](http://services.brieflands.com/cdn/serve/313ea/b4c6bf636867020bf486fab71b01db0d93757c83/jjhs-8-2-35108-i004-preview.png)
5. Discussion
The current study was the first to investigate the treatment of PCP containing petrochemical high-TDS wastewater by the persulfate activation method, which involves advanced oxidation processes. The results showed that ZVI could activate persulfate to degrade recalcitrant organic contaminants, such as PCP. Under the given experimental conditions, within 120 minutes of reaction time, the removal efficiency of PCP was almost 70%, at an initial PS concentration of 15.0 mM in the presence of 1.0 g/L Fe° at pH = 5.0 ± 0.1 and T = 25°C. Moreover, decreasing the initial pH and moderately increasing contents of Fe° and persulfate enhanced the degradation efficiency of PCP. ZVI exhibit good stability and reusability, and they can be separated from solutions with a magnet and reused three times. Therefore, the activation of persulfate by Fe° is a cost-effective technology that can remediate industrial wastewater. This strategy is recommended for organic wastewater treatment.
Acknowledgements
References
-
1.
Sze MF, McKay G. Enhanced mitigation of para-chlorophenol using stratified activated carbon adsorption columns. Water Res. 2012;46(3):700-10. [PubMed ID: 22154109]. https://doi.org/10.1016/j.watres.2011.11.039.
-
2.
Aslam M, Soomro MT, Ismail IM, Salah N, Gondal MA, Hameed A. Sunlight mediated removal of chlorophenols over tungsten supported ZnO: electrochemical and photocatalytic studies. Journal of Environmental Chemical Engineering. 2015;3(3):1901-11.
-
3.
Kusic H, Koprivanac N, Bozic AL. Treatment of chlorophenols in water matrix by UV/ferrioxalate system: Part I. Key process parameter evaluation by response surface methodology. Desalination. 2011;279(1):258-68.
-
4.
Liang J, Peng X, Yin D, Li B, Wang D, Lin Y. Screening of a microbial consortium for highly simultaneous degradation of lignocellulose and chlorophenols. Bioresour Technol. 2015;190:381-7. [PubMed ID: 25974352]. https://doi.org/10.1016/j.biortech.2015.04.105.
-
5.
Jia H, Wang C. Dechlorination of chlorinated phenols by subnanoscale Pd(0)/Fe(0) intercalated in smectite: pathway, reactivity, and selectivity. J Hazard Mater. 2015;300:779-87. [PubMed ID: 26313617]. https://doi.org/10.1016/j.jhazmat.2015.08.017.
-
6.
Martins LFG, Parreira MCB, Ramalho JPP, Morgado P, Filipe EJM. Prediction of diffusion coefficients of chlorophenols in water by computer simulation. Fluid Phase Equilibria. 2015;396:9-19.
-
7.
Zhang L, Zhang B, Wu T, Sun D, Li Y. Adsorption behavior and mechanism of chlorophenols onto organoclays in aqueous solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2015;484:118-29.
-
8.
Boncz MA, Bruning H, Rulkens WH, Sudholter EJR, Harmsen GH, Bijsterbosch JW. Kinetic and mechanistic aspects of the oxidation of chlorophenols by ozone. Water Science and Technology. 1997;35(4):65-72.
-
9.
Havlikova L, Satinsky D, Solich P. Aspects of decontamination of ivermectin and praziquantel from environmental waters using advanced oxidation technology. Chemosphere. 2016;144:21-8. [PubMed ID: 26344145]. https://doi.org/10.1016/j.chemosphere.2015.08.039.
-
10.
Catrinescu C, Arsene D, Teodosiu C. Catalytic wet hydrogen peroxide oxidation of para-chlorophenol over Al/Fe pillared clays (AlFePILCs) prepared from different host clays. Applied Catalysis B: Environmental. 2011;101(3):451-60.
-
11.
Hao X, Zhou M, Xin Q, Lei L. Pulsed discharge plasma induced Fenton-like reactions for the enhancement of the degradation of 4-chlorophenol in water. Chemosphere. 2007;66(11):2185-92. [PubMed ID: 17166558]. https://doi.org/10.1016/j.chemosphere.2006.08.037.
-
12.
Hu P, Long M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Applied Catalysis B: Environmental. 2016;181:103-17. https://doi.org/10.1016/j.apcatb.2015.07.024.
-
13.
Kralik P, Kusic H, Koprivanac N, Bozic AL. Degradation of chlorinated hydrocarbons by UV/H 2 O 2: the application of experimental design and kinetic modeling approach. Chemical engineering journal. 2010;158(2):154-66.
-
14.
Chen X, Murugananthan M, Zhang Y. Degradation of p-Nitrophenol by thermally activated persulfate in soil system. Chemical Engineering Journal. 2016;283:1357-65.
-
15.
Xie P, Ma J, Liu W, Zou J, Yue S, Li X, et al. Removal of 2-MIB and geosmin using UV/persulfate: contributions of hydroxyl and sulfate radicals. Water Res. 2015;69:223-33. [PubMed ID: 25486622]. https://doi.org/10.1016/j.watres.2014.11.029.
-
16.
Zhao YS, Sun C, Sun JQ, Zhou R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe 3 O 4 nanoparticles process. Separation and Purification Technology. 2015;142:182-8.
-
17.
Rodriguez S, Vasquez L, Costa D, Romero A, Santos A. Oxidation of Orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere. 2014;101:86-92. [PubMed ID: 24439838]. https://doi.org/10.1016/j.chemosphere.2013.12.037.
-
18.
Weng CH, Tsai KL. Ultrasound and heat enhanced persulfate oxidation activated with Fe(0) aggregate for the decolorization of C.I. Direct Red 23. Ultrason Sonochem. 2016;29:11-8. [PubMed ID: 26584979]. https://doi.org/10.1016/j.ultsonch.2015.08.012.
-
19.
Cai C, Zhang H, Zhong X, Hou L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of Orange II in water. J Hazard Mater. 2015;283:70-9. [PubMed ID: 25262480]. https://doi.org/10.1016/j.jhazmat.2014.08.053.
-
20.
Sharma J, Mishra IM, Dionysiou DD, Kumar V. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chemical Engineering Journal. 2015;276:193-204.
-
21.
Tang D, Zhang G, Guo S. Efficient activation of peroxymonosulfate by manganese oxide for the degradation of azo dye at ambient condition. J Colloid Interface Sci. 2015;454:44-51. [PubMed ID: 26002338]. https://doi.org/10.1016/j.jcis.2015.05.009.
-
22.
Cao J, Zhang WX, Brown DG, Sethi D. Oxidation of lindane with Fe (II)-activated sodium persulfate. Environmental Engineering Science. 2008;25(2):221-8.
-
23.
Chen WS, Huang CP. Mineralization of aniline in aqueous solution by electrochemical activation of persulfate. Chemosphere. 2015;125:175-81. [PubMed ID: 25576128]. https://doi.org/10.1016/j.chemosphere.2014.12.053.
-
24.
Deng D, Peng L, Guan M, Kang Y. Impact of activation methods on persulfate oxidation of methyl tert-butyl ether. J Hazard Mater. 2014;264:521-8. [PubMed ID: 24246442]. https://doi.org/10.1016/j.jhazmat.2013.10.042.
-
25.
Thomas JM, Hernandez R, Kuo CH. Single-step treatment of 2,4-dinitrotoluene via zero-valent metal reduction and chemical oxidation. J Hazard Mater. 2008;155(1-2):193-8. [PubMed ID: 18166266]. https://doi.org/10.1016/j.jhazmat.2007.11.073.
-
26.
Wang X, Wang L, Li J, Qiu J, Cai C, Zhang H. Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Separation and Purification Technology. 2014;122:41-6.
-
27.
Deng J, Shao Y, Gao N, Deng Y, Tan C, Zhou S. Zero-valent iron/persulfate (Fe0/PS) oxidation acetaminophen in water. International Journal of Environmental Science and Technology. 2014;11(4):881-90.
-
28.
Andrew D. Standard Method for the Examination of Water and Wastewater. 21 ed. Washington DC: American Public Health Association; 2005.
-
29.
Zhao J, Zhang Y, Quan X, Chen S. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature. Separation and Purification Technology. 2010;71(3):302-7.
-
30.
Oh SY, Kim HW, Park JM, Park HS, Yoon C. Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater. 2009;168(1):346-51. [PubMed ID: 19285795]. https://doi.org/10.1016/j.jhazmat.2009.02.065.
-
31.
Xu XR, Li XZ. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Separation and purification technology. 2010;72(1):105-11.