Abstract
Background:
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, and neurodegenerative autoimmune disease of the central nervous system (CNS). In the disease, the immune system attacks myelin sheath around neurons. The pathogenesis of this disease is still unknown, but there is evidence of complex interactions of both environmental and genetic factors. The recent evidence suggests that HLA class I may also contribute to MS and the negative association and protective role of HLA-A*02 with MS is confirmed. The current study aimed at investigating the association between HLA-A*02 with MS in Khuzestan province, Iran.Methods:
The current study was conducted on 200 patients with MS and 200 healthy controls from Khuzestan Province. The male to female ratio was 1:4. HLA typing was performed by polymerase chain reaction (PCR) amplification with sequence-specific primers (PCR-SSP) method. The association between HLA-A*02 and MS was evaluated with SPSS version 21 using chi-square test.Results:
The most abundant type of MS in the current study participants was RRMS with the frequency of 87.93%, and the remaining 20% had progressive relapsing (PR), primary progressive (PP), and secondary progressive (SP) MS. There was a significant decrease in the frequency of HLA-A*02 in patients with MS (29%) in comparison with the healthy control (54%) and negative association of HLA-A*02 with MS (P < 0.0001, odds ratio (OR) = 0.356) was observed. The average of EDSS was 1.9 and most of the patients (about 60.5%) had EDSS of 1 - 3. There was no significant statistical association between HLA-A*02 and the variables of gender, ethnicity, EDSS grade, and type of MS in the study participants.Conclusions:
According to the current study results, HLA–A*02 genotype decreased the risk of MS and had a protective role against multiple sclerosis in Khuzestan Province.Keywords
1. Background
Multiple sclerosis (MS) is a chronic inflammatory neurodegenerative disorder in which the insulating coats of nerve cells in the brain and spinal cord are damaged (1). This damage disrupts communications in the nervous system resulting a wide range of signs and symptoms (2, 3). Some of the clinical features include visual problems such as nystagmus, optic neuritis or double vision, acute or chronic paint, muscle spasm, and difficulty in movement (4). There are significant differences in the occurrence of MS in various geographical regions. About 30 years ago, Kurtzke (5, 6) classified geographical areas in 3 regions according to MS prevalence: (1) high > 30/100,000, (2) intermediate (5 - 25/100,000), and (3) low risk < 5/100,000. Also, he reported that the prevalence of MS increases with increasing latitude (7).
The age of onset is typically between 20 - 40 years and females are at risk 3 times more than males (8, 9). Approximately, 350,000 individuals in the United States and 2.5 million individuals globally are affected by MS (10). The worldwide medium prevalence increased from 30 (in 2008) to 33 per 100,000 individuals (in 2013) (11). Recent investigations showed that the prevalence of MS in Iran increased significantly (12) and it was 45/100,000 individuals (in 2011) (13). In the recent studies, the prevalence of MS in Khuzestan province, South-West of Iran, was 15 - 18.50 per 100,000 people (14, 15). The clinical studies described 4 types of MS: 1) About 85% of the affected patients are diagnosed with relapsing-remitting (RRMS) type of MS, a relapse phase followed by remission phase (16); 2) The second most frequent type of MS is secondary progressive MS (SPMS) that occurs in about 30% of the patients (1); 3) The third type of MS that affects 10% - 15% of people with MS is called primary progressive MS (PPMS) (17); 4) Progressive-relapsing MS (PRMS) occurs in 5% of cases and has overlap with PPMS and SPMS (7). The Kurtzke expanded disability status scale (EDSS) is a method to quantify disability in multiple sclerosis. The EDSS quantifies disability in 8 functional systems consisting of pyramidal, brainstem, cerebral, sensory, bowel and bladder, visual, cerebellar, etc., and allows neurologists to assign a functional system score (FSS) in each of such cases. The range of EDSS varies from 0 to 10 (0 describes no disability and 10 describes death due to MS) (18).
MS is a multifactorial disease and there are different factors involved in the pathogenesis of the disease. Genetic components play an important role in MS disease, either by themselves or in communication with environmental factors (19). Studies about twins revealed 25% - 30% concordance rate in monozygotic twins compared with dizygotic twins with 5% concordance (20). Monozygotic twins are by birth genetically identical and 30% concordance rate in monozygotic twins indicates that additional factors such as virus infections, diet, exposure to sunlight, and geographical distribution can be involved in trigger and development of multiple sclerosis (21, 22). One of the most important genetic factors in pathogenesis of MS is MHC (major histocompatibility complex) genes located in short arm of the chromosome 6 (6p21.3) (2) responsible for 20% - 60% of genetic factors that contribute to the susceptibility to MS (23). This region of genome is extensively studied due to its important roles in autoimmune, infectious and inflammatory diseases, and in transplantation (24).
The function of MHC molecules is to bind peptide antigens derived from pathogens and present them on the cell surface to be identified by the related T-cells (25). The mechanisms of association of MHC with the disease are not clearly understood. One long-held view suggests a breakdown in immunological tolerance to self-antigens through aberrant class II presentation of self or foreign peptides to autoreactive T-lymphocytes. Thus, it seems likely that specific MHC class II alleles determine the targeting of particular autoantigens resulting in disease-specific associations (24). The MHC genes include HLA (human leukocyte antigen). There are 3 classes of HLA I-III. Class I and II gene products are highly polymorphic cell surface glycoproteins involved in discrimination of self from non-self by the immune system. Class I includes HLA-A, B, and C genes and class II includes HLA-DP, DQ, and DR (25). Class III region includes genes encoding 4 serum proteins of the complement system, i e, C2, Bf, C4A, and C4B, and steroide 21-hydroxylase (26). Association of HLA genes with MS was studied about 40 years ago and the protective role of HLA-A*02, one of the major types of HLA-A gene, in MS was confirmed (23).
The current study aimed at investigating the effect and association of HLA-A*02 antigen with genetic susceptibility/resistance to MS, and determining the impact of HLA-A*02 on gender, ethnicity, type of MS, and EDSS in patients with MS in Khuzestan Province, Iran.
2. Methods
2.1. Study Groups
There were 2 groups in the current study. One group included 200 patients with MS from Khuzestan Province that their diseases were diagnosed according to McDonald criteria (recommended diagnostic criteria for multiple sclerosis) (27) by the expert neurologist, and the other group included 200 healthy controls that matched by the patients group for age and gender. The healthy group did not have any autoimmune or inflammatory diseases. The mean age of the patients was 31.6 years, ranged 16 - 50; in the healthy control group, the mean age was 30.9 years. Genomic DNA extraction was performed by salting out method as follows: 2 - 10 mL blood sample in ethylenediaminetetraacetic acid (EDTA) was transferred to a 15-mL tube and centrifuged at 13,000 g for 10 minutes. The supernatant was discarded and a solution of 1% SDS, 5 mM EDTA, 10 mM Tris-HCl, and proteinase K (10 mg/mL) was added to the precipitate and incubated for 2 hours at 37°C. Then, saturated NaCl was added to the tube, shaken well and centrifuged at 13,000 g for 5 minutes. The upper aqueous layer was transferred to a clean tube and 0.7X isopropanol was added. The precipitated DNA was collected by centrifugation at 13000 g for 2 minutes and 2X of 100% ethanol was added to the precipitate. After centrifugation at 13000 g for 2 minutes, the pellet was dried until all the ethanol was evaporated off. Then, the pellet was dissolve in an appropriate amount of sterile water.
2.2. HLA Typing
The HLA-A*02 typing was investigated by the polymerase chain reaction (PCR) amplification method on the extracted genomic DNA with sequence-specific primers (PCR-SSP). Each PCR-SSP reaction was deemed to have worked if the internal control amplification was observed. Myelin oligodendrocyte glycoprotein (MOG) gene was used as the internal control. A pair of primers was used as HLA-A*02 primers for amplification of related HLA antigen. Also, a pair of MOG primers was used as the internal positive control (Table 1). The PCR solutions were prepared as follows: 100 µg genomic DNA, 200 μM dNTP, 1.5 mM MgCl2, 2.5 units SuperTaq polymerase (Genfanavaran, Iran), and 25 pmol of each primer. PCR was performed in a total volume of 25 μL in 35 cycles. PCR conditions were as follows: 95°C for 2 minutes; 30 cycles of 95°C for 30 seconds, 59°C for 30 seconds, 72°C for 30 seconds; and then 72°C for 5 minutes. Finally, several samples were randomly sequenced to validate the results.
Primer Sequences
| Name | Primer Sequence | Tm, °C |
|---|---|---|
| HLA-A*02-F | 5′-TCCTCGTCCCCAGGCTCT-3′ | 60.5 |
| HLA-A*02-R | 5′-CCCCAGGTCCACTCGGTG-3′ | 62.8 |
| MOG-V142-F | 5′-GGGACCAATTCTGTGTCCC -3′ | 60 |
| MOG-V142-R | 5′-TGAACCCAGAAGTCACTC ACA -3′ | 59 |
The presence of HLA-A*02 antigens were detected by the appearance of 249-bp bands on gel electrophoresis. The band size of MOG gene was 356 bp, which should be appeared in all samples (Figure 1). The frequency of the HLA-A*02 was determined as a percentage and the significance association of HLA-A*02 with MS was evaluated with SPSS version 21 statistical software using chi-square test. P values less than 0.05 were considered the level of significance. Also, correlations between HLA-A*02 and EDSS , type of MS, gender, ethnicity, and some clinical symptoms of MS were investigated.
Gel electrophoresis. Column 1 is a 100-bp marker. Column 2, 3 are negative controls, columns 4, 5, 6, and 8 are positive samples, columns 7 and 9 are negative samples. Control MOG gene PCR product was 365 bp and HLA-A*02 PCR product was 249 bp.
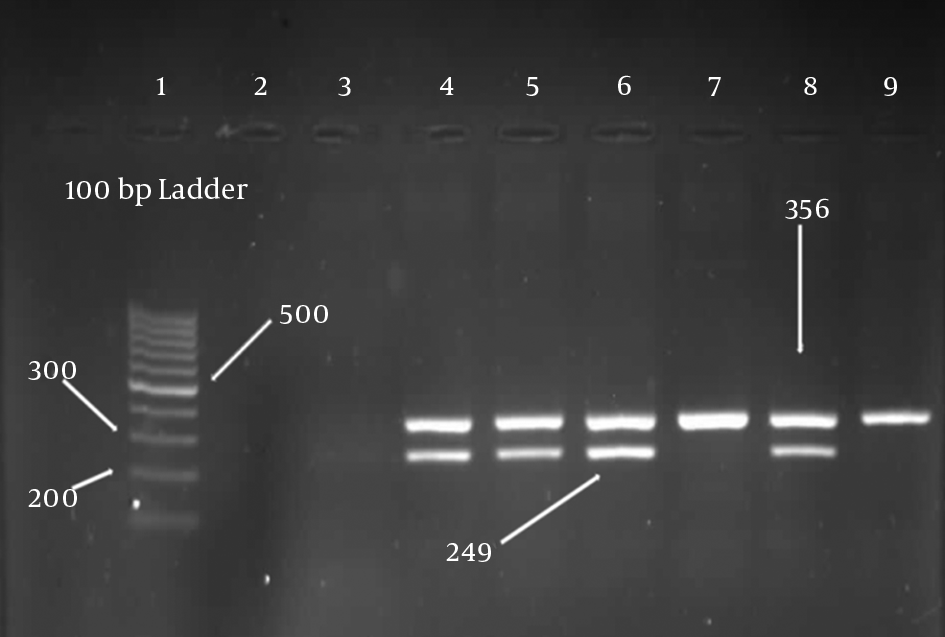
3. Results
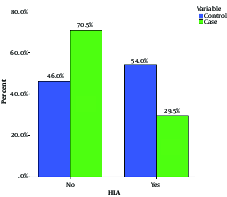
The male to female ratio in patients was 1:4 (Table 2) and the most abundant type of MS in the patient group was RRMS with the frequency of 87.93% (Table 3). Chi-square, statistical analysis, and the Pearson chi-square models were used to determine the association between HLA-A*02 and different variables in patients with MS. As shown in Table 4, there was a significant decrease in the frequency of HLA-A*02 in patients with MS (29%) in comparison with those of the healthy controls (54%); and a negative association between HLA-A*02 and MS (P < 0.0001, odds ratio (OR) = 0.356) was also observed. The data indicated the protective role of HLA-A*02 against MS in Khuzestan Province. The average of EDSS was 1.9 and most of the patients (about 60.5%) had EDSS 1 - 3 (Table 5). Two ethnic groups were studied, Arab and Persian. The number of Arab and Persian individuals was 44 and 72, respectively (37.9% vs. 62.1%) (Table 6). There was no significant statistical association between HLA-A*02 and variables of gender, ethnicity, EDSS, and type of MS in the current study. Some of the clinical symptoms and their frequencies are shown in Table 7 and Figure 2.
| Gender | HLA-A*02 | P Value | |
|---|---|---|---|
| No | Yes | ||
| Male | 28 | 13 | 0.723 |
| Female | 106 | 43 | |
| Total | 134 | 56 | |
| HLA-A*02 | Disease | P Value | ||
|---|---|---|---|---|
| RRMS | Other | Total | ||
| No | ||||
| Count | 104 | 17 | 121 | |
| % of total | 59.8% | 9.8% | 69.5% | |
| Yes | ||||
| Count | 49 | 4 | 53 | 0.226 |
| % of total | 28.2% | 2.3% | 30.5% | |
| Total | ||||
| Count | 153 | 21 | 174 | |
| % of total | 87.9% | 12.1% | 100.0% | |
| P Value | Population | HLA-A*02 | |
|---|---|---|---|
| Control | Case | ||
| < 0.0001 | 108 | 59 | + |
| 92 | 141 | - | |
| 200 | 200 | total | |
| EDSS | HLA-A*02 | Total | |
|---|---|---|---|
| No | Yes | ||
| 0 | |||
| Count | 18 | 8 | 26 |
| % of total | 10.5% | 4.7% | 15.1% |
| 1 - 3 | |||
| Count | 81 | 40 | 121 |
| % of total | 47.1% | 23.3% | 70.3% |
| 3 - 5 | |||
| Count | 15 | 3 | 18 |
| % of total | 8.7% | 1.7% | 10.5% |
| > 5 | |||
| Count | 5 | 2 | 7 |
| % of total | 2.9% | 1.2% | 4.1% |
| Total | |||
| Count | 119 | 53 | 172 |
| % of total | 69.2% | 30.8% | 100.0% |
| HLA-A*02 | P Value | ||
|---|---|---|---|
| No | Yes | ||
| Arab | 32 | 12 | 0.70 |
| Persian | 50 | 22 | |
| Total | 82 | 34 | |
Relationship Between HLA-A*02 and Some Clinical Symptoms
| Symptom | HLA-A*02, % | P Value | |
|---|---|---|---|
| No | Yes | ||
| Headache | 51.7 | 24.6 | 0.716 |
| Blurred vision | 44.7 | 24.3 | 0.479 |
| Constipation | 49.3 | 18.7 | 0.539 |
| Limbs numbness | 43.22 | 22.03 | 0.43 |
| Frequent urination | 40.6 | 17.4 | 0.415 |
Frequency of HLA-A*02 in Case and Control Groups
4. Discussion
According to the studies by Kurtezke, Iran is located at the low-risk area for MS. However, recent studies revealed that multiple sclerosis is increasing in Iran. There are some explanations for the increasing pattern of MS in the Middle-East and also Iran. One of the most important causes may be the age of the population in Iran. Iran has a very young population, with the majority age range of 15-30 years. The fact that the disease is more prevalent in young adults means that age may be an important factor for the rise in the prevalence (13). The relationship between vitamin D deficiency and MS is reported by several studies. Accordingly, the increase in the prevalence of vitamin D deficiency may be another explanation for the rise in the prevalence of MS in Iran. This may be the consequence of the rise in the use of cosmetics and sunscreen creams, or exacerbation of air pollution, especially in industrial areas such as Isfahan, Tehran, and Fars provinces, all of which have the highest rates of MS cases in Iran (12).
Therefore, Iran can be accounted as an intermediate risk area. For this reason, investigation on MS is necessary in Iran. Frequencies of class II of HLA include HLA-DQB1*0602, -DRB1*1501, and -DRB5*01 variants in the MS population of Khuzestan Province were already reported; association of HLA-DRB1*1501 and no association of HLA DRB5*01 and HLA-DQB1*0602 with MS in the population were observed (28-30). The current study investigated the association between HLA-A*02, an important type of HLA-A gene of HLA class I in the diagnosis or pathogenesis of MS, and MS in Khuzestan Province, Iran.
It is believed that MS is predominant among females compared to males (31-33). In the current study the female to male ratio was 4:1, which confirmed that MS was more common in females than in males. In a review in 2013 from Iran, the reported ratio ranged 1.8 to 3.8 (34). Also, other study from Khuzestan Province reported that most patients with MS (70.5%) were female (35). Moreover, the results of the current study revealed that the RR was the predominant type of MS. Recent studies from Iran also reported that the majority of patients with MS (80.1%) and (92.1%) had RR in Qom and Khuzestan provinces, respectively (35, 36).
In the current study, most of the patients had EDSS 1 - 3. These results were in agreement with other data from investigating the epidemiology of MS in Khuzestan province (85% of the patients had mild disease (0 - 3) (35).
Kobelt showed that EDSS score was different between countries (Germany 3.8 (± 2.3), Netherlands 3.9 (± 2.2), Switzerland 4.5 (± 2.4), and the UK 5.1 (± 2) (37).
The current study found that HLA-A*02 had a protective role against MS in Khuzestan Province (P < 0.0001, OR= 0.356, 95%CI: 0.236 - 0.538). This finding was in line with the studies in other populations such as Portuguese (38), Italian (39), Swedish (40), Italian-UK (41), and African-Americans (42). All of the investigations confirmed the negative association of HLA-A*02 with MS. In the study on Portuguese population, the authors indicated the protective role of HLA-A*02 allele in the Portuguese patients with MS (P = 0.001, OR = 0.53) (38). However, the results of the current study did not match with those of other studies in Iran. In a study in Tehran, there was no association between HLA-A*02 and MS. In the study, the frequencies of HLA-A*02 were 36.7% and 35% in the case and control groups, respectively (43). In a study in Kerman, the results did not confirm the negative association of HLA antigen with MS, but a protective role of HLA-A*02 for some of the clinical symptoms such as spasticity was observed (44). In a genome-wide association study of the A and B locus of the HLA system, Lotfi et al. did not report the protective role of HLA-A*02 against MS (45). Also, in a study by Ramadan et al., on Arab population, there was no association between MS and HLA-A*02; but similar to the study in Kerman, they reported a protective role of HLA-A*02antigen against muscular dysfunction in Arab patients with MS (46).
The current study investigated the differences of HLA-A*02 frequency between Arab and Persian populations in Khuzestan Province; there was no significant differences in the frequency of this HLA type between the 2 populations (Table 6). Also, there was no significant differences in HLA-A*02 frequency between males and females in Khuzestan Province (Table 2). Furthermore, the effect of HLA-A*02 on some common clinical signs was examined. No protective role of this HLA type on the clinical symptoms of MS was observed (Table 7). Some of the clinical features of MS were more frequent in the current study cases in comparison with those of other studies including headache, blurred vision, constipation, limbs numbness, and frequent urination, and the reason can be attributed to genetic background of Khuzestan Population. It can be concluded that HLA–A*02 genotype decreased the risk of MS and had a protective role against MS in Khuzestan Province. It is recommended to evaluate this allele in MS patients in the other provinces of Iran for more documented results.
Acknowledgements
References
-
1.
Zuvicha R, McCauleyb JL, Pericak-Vanceb MA. Genetics and pathogenesis of multiple sclerosis. Seminars Immunol. 2009;21:328-33.
-
2.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502-17. [PubMed ID: 18970977]. https://doi.org/10.1016/S0140-6736(08)61620-7.
-
3.
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221-31. [PubMed ID: 11955556]. https://doi.org/10.1016/S0140-6736(02)08220-X.
-
4.
Roxburgh RHSR, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple Sclerosis Severity Score Using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144-51.
-
5.
Kurtzke JF. Epidemiologic contributions to multiple sclerosis: an overview. Neurology. 1980;30(7 Pt 2):61-79. [PubMed ID: 6993993].
-
6.
Kurtzke JF. A reassessment of the distribution of multiple sclerosis. Acta Neurol Scand. 1975;51(2):137-57. [PubMed ID: 1114878].
-
7.
Benamer HT, Ahmed ES, Al-Din AS, Grosset DG. Frequency and clinical patterns of multiple sclerosis in Arab countries: a systematic review. J Neurol Sci. 2009;278(1-2):1-4. [PubMed ID: 19135686]. https://doi.org/10.1016/j.jns.2008.12.001.
-
8.
Weiner HL, Stankiewicz JM. Multiple sclerosis: Diagnosis and therapy. John Wiley & Sons; 2012.
-
9.
Multiple sclerosis Trust. Multiple sclerosis information for health and social care professionals (fourth edition). Hertfordshire: Multiple sclerosis Trust; 2011.
-
10.
Hauser SL, Goodin DS. Multiple sclerosis and other demyelinating diseases (chapter 380). In: Fauci AS, Braunwald E, Kasper DL, editors. Harrison's Principles of Internal Medicine. 17th ed. Volumes I and II. New York: The McGraw-Hill Companies, Inc; 2008. p. 2611-4.
-
11.
Multiple Sclerosis International Federation. Atlas of MS. 2013.
-
12.
Etemadifar M, Janghorbani M, Shaygannejad V, Ashtari F. Prevalence of multiple sclerosis in Isfahan, Iran. Neuroepidemiology. 2006;27(1):39-44. [PubMed ID: 16804333]. https://doi.org/10.1159/000094235.
-
13.
Izadi S, Nikseresht A, Sharifian M, Sahraian MA, Hamidian Jahromi A, Aghighi M, et al. Significant increase in the prevalence of multiple sclerosis in iran in 2011. Iran J Med Sci. 2014;39(2):152-3. [PubMed ID: 24644387].
-
14.
Radmehr M, Meghdadi S, Bahmanzadeh M, Sabbagh S. Prevalence, Demographics and Clinical Characteristics of Multiple Sclerosis in North of Khuzestan Province, Iran. Jentashapir J Health Res. 2015;6(5). https://doi.org/10.17795/jjhr-23831.
-
15.
Sharafaddinzadeh N, Moghtaderi A, Majdinasab N, Dahmardeh M, Kashipazha D, Shalbafan B. The influence of ethnicity on the characteristics of multiple sclerosis: a local population study between Persians and Arabs. Clin Neurol Neurosurg. 2013;115(8):1271-5. [PubMed ID: 23273383]. https://doi.org/10.1016/j.clineuro.2012.11.027.
-
16.
Samkoff LM, Goodman AD. Symptomatic management in multiple sclerosis. Neurol Clin. 2011;29(2):449-63. [PubMed ID: 21439453]. https://doi.org/10.1016/j.ncl.2011.01.008.
-
17.
The National Collaborating Centre for Chronic conditions. Multiple Sclerosis: National clinical guideline for diagnosis and management in primary and secondary care. 2004. Available from: http://www.nice.org.uk/nicemedia/live/10930/46699/46699.
-
18.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-52. [PubMed ID: 6685237].
-
19.
Jafari N, Broer L, Hoppenbrouwers IA, van Duijn CM, Hintzen RQ. Infectious mononucleosis-linked HLA class I single nucleotide polymorphism is associated with multiple sclerosis. Mult Scler. 2010;16(11):1303-7. [PubMed ID: 20736246]. https://doi.org/10.1177/1352458510376778.
-
20.
Hawkes CH, Macgregor AJ. Twin studies and the heritability of MS: a conclusion. Multiple Sclerosis J. 2009;15(6):661-7.
-
21.
Oksenberg JR, Baranzini SE, Sawcer S, Hauser S. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genetics. 2008;9(7):516.
-
22.
Hoffjan Sand Akkad D. The genetics of multiple sclerosis: An update. Molecular and Cellular Probes. 2010.
-
23.
Gourraud PA, Harbo HF, Hauser SL, Baranzini SE. The genetics of multiple sclerosis: an up-to-date review. Immunol Rev. 2012;248(1):87-103. [PubMed ID: 22725956]. https://doi.org/10.1111/j.1600-065X.2012.01134.x.
-
24.
Fernando MMA, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Gene. 2008;4(4). e1000024.
-
25.
Travers P, Walport M, Capra JD. Immunobiology: the immune system in health and disease. Current Biology Ltd. 1999.
-
26.
Volanakis JE, Zhu ZB, Schaffer FM, Macon KJ, Palermos J, Barger BO, et al. Major histocompatibility complex class III genes and susceptibility to immunoglobulin A deficiency and common variable immunodeficiency. J Clin Invest. 1992;89(6):1914-22. [PubMed ID: 1351062]. https://doi.org/10.1172/JCI115797.
-
27.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-7. [PubMed ID: 11456302].
-
28.
Pakdehi T, Shafiei M, Galehdari H. HLA DRB5* 01 Association Survey with Multiple Sclerosis in Khuzestan Province of Iran. Zahedan J Res Med Sci. 2017;19(8).
-
29.
Zabihi R, Galehdari H, Shafiee M, Kazeminejad SR, Alavi SM. Analysis of HLA-DQB1*0602 in Multiple Sclerosis Patients in Khuzestan Province, Iran. Arch Iran Med. 2015;18(10):698-702. [PubMed ID: 26443252].
-
30.
Delfan N, Galehdari H, Kazeminejad SR, Shafiei M, Zabihi R, Majdinasab N. Analysis of HLA-DRB1*1501 in Multiple Sclerosis Patients in Khuzestan Province, Iran. Zahedan J Res Med Sci. 2017;19(3). https://doi.org/10.5812/zjrms.5647.
-
31.
Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001;22(2):117-39. [PubMed ID: 11603614].
-
32.
Moreira MA, Felipe E, Mendes MF, Tilbery CP. [Multiple sclerosis: descriptive study of its clinical forms in 302 cases]. Arq Neuropsiquiatr. 2000;58(2B):460-6. [PubMed ID: 10920407].
-
33.
Kalanie H, Gharagozli K, Kalanie AR. Multiple sclerosis: report on 200 cases from Iran. Mult Scler. 2003;9(1):36-8. [PubMed ID: 12617266]. https://doi.org/10.1191/1352458503ms887oa.
-
34.
Etemadifar M, Sajjadi S, Nasr Z, Firoozeei TS, Abtahi SH, Akbari M, et al. Epidemiology of multiple sclerosis in Iran: a systematic review. Eur Neurol. 2013;70(5-6):356-63. [PubMed ID: 24192707]. https://doi.org/10.1159/000355140.
-
35.
Torabipour A, Asl ZA, Majdinasab N, Ghasemzadeh R, Tabesh H, Arab M. A study on the direct and indirect costs of multiple sclerosis based on expanded disability status scale score in khuzestan, iran. Int J Prev Med. 2014;5(9):1131-8. [PubMed ID: 25317296].
-
36.
Rezaali S, Khalilnezhad A, Naser Moghadasi A, Chaibakhsh S, Sahraian MA. Epidemiology of multiple sclerosis in Qom: Demographic study in Iran. Iran J Neurol. 2013;12(4):136-43. [PubMed ID: 24250923].
-
37.
Kobelt G, Berg J, Lindgren P, Gerfin A, Lutz J. Costs and quality of life of multiple sclerosis in Switzerland. Eur J Health Econ. 2006;7 Suppl 2:S86-95. [PubMed ID: 17310338]. https://doi.org/10.1007/s10198-006-0383-9.
-
38.
Silva AM, Bettencourt A, Pereira C, Santos E, Carvalho C, Mendonca D, et al. Protective role of the HLA-A*02 allele in Portuguese patients with multiple sclerosis. Mult Scler. 2009;15(6):771-4. [PubMed ID: 19482867]. https://doi.org/10.1177/1352458509104588.
-
39.
Bergamaschi L, Leone MA, Fasano ME, Guerini FR, Ferrante D, Bolognesi E, et al. HLA-class I markers and multiple sclerosis susceptibility in the Italian population. Genes Immun. 2010;11(2):173-80. [PubMed ID: 19907433]. https://doi.org/10.1038/gene.2009.101.
-
40.
Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55(2):140-8. [PubMed ID: 10746785].
-
41.
Bergamaschi L, Ban M, Barizzone N, Leone MA, Ferrante D, Fasano ME, et al. Association of HLA class I markers with multiple sclerosis in the Italian and UK population: evidence of two independent protective effects. J Med Gene. 2011:jmg. 2010.080721.
-
42.
Isobe N, Gourraud PA, Harbo HF, Caillier SJ, Santaniello A, Khankhanian P, et al. Genetic risk variants in African Americans with multiple sclerosis. Neurology. 2013;81(3):219-27. [PubMed ID: 23771490]. https://doi.org/10.1212/WNL.0b013e31829bfe2f.
-
43.
Kalanie H, Kamgooyan M, Sadeghian H, Kalanie AR. Histocompatibility antigen (HLA) associations with multiple sclerosis in Iran. Mult Scler. 2000;6(5):317-9. [PubMed ID: 11064440]. https://doi.org/10.1177/135245850000600504.
-
44.
Zabihi A, Ebrahimi H, Rashidfarrokhi F, Ramazani A. Investigating the relationship between MS disease and its symptoms and class I leukocyte antigens in Kerman city. 2005.
-
45.
Lotfi J, Nikbin B, Derakhshan I, Aghai Z, Ala F. Histocompatibility antigens (HLA) in multiple sclerosis in Iran. J Neurol Neurosurg Psychiatry. 1978;41(8):699-701. [PubMed ID: 681957].
-
46.
Ramadan MA, Ali IR, Mohamed DH, Abboud H, El Gendy W. Study of the association between the human leukocyte antigen class i molecules and remitting relapsing multiple sclerosis ms. Bull Alex Fac Med. 2008;44(4).
