Abstract
Background:
Nowadays, water pollution by toxic elements has become a universal environmental issue. Lead, as a toxic element, is approximately enhanced in the environment by industrial development. The potential risk of lead to health has attracted the interest of researchers to eliminate it from contaminated water.Objectives:
In the present study, a dithizone-modified bagasse adsorbent, used for removing lead ions from aqueous solutions, was successfully prepared.Methods:
The prepared adsorbent was characterized by fourier-transform infrared (FT-IR) and X-ray diffractometer (XRD) techniques. The adsorptive properties of adsorbent were studied at room temperature. Different parameters like pH value, solution concentration, adsorption time, and amount of adsorbent were optimized.Results:
Results show that the adsorption of lead on dithizone-modified bagasse fitted well to the Freundlich isotherm equation following the Langmuir isotherm. The maximum removal efficiency was obtained to be more than 99.5% in a short contact time (1.0 minutes) at pH 6. The loading capacity was studied and obtained to be 37.20 mg g-1. The adsorption process was replicated for 4 times without a considerable loss in its efficiency (> 95%).Conclusions:
High efficiency at a short time, recyclability, and biodegradability are the main advantages of this adsorbent. The proposed method is cost-effective and environmentally friendly.Keywords
1. Background
Water pollution by toxic metals associated with industrial development has become a worldwide environmental problem in 21st century. Heavy metals release into the environment through many different ways, such as industrial processes, agriculture, and urban life. The heavy metals are easily transferred to human bodies through the food chain (1). Lead, which is one of the heavy metals present in the environment, is produced from various industrial operations, such as metal smelting, battery manufacturing, mining, alloy formation, and fossil fuels (2). Generic acute and chronic symptoms caused by lead exposure are neurosis, behavioral difficulties, dyspepsia and osteoporosis, disturbance to the immune system, and disorder to some organs including heart, liver, and kidneys (3). Thus, due to the risk of lead to health, the elimination of Pb (II) from contaminated water is essential for controlling wastewater pollution and contamination of the environment.
In the last decades, various methods have been utilized for the treatment of water, such as chemical precipitation, solvent extraction, membrane filtration, ion exchange, and coagulation (4). Traditional water treatment methods are deficient with some reasons including high cost, non-selectivity, time consuming, and complicated operation (5).
Based on the use of industrial wastes, new solid phase extraction (SPE) techniques have been introduced. Several studies have been performed on industrial by-products such as fly ash, iron slags, titanium oxide, sawdust, and bagasse fly ash. The mentioned leftover by-products can be physically and/or chemically modified to improve their adsorption efficiencies in order to remove heavy metals from contaminated water (6-10).
Many cane sugar factories had to face adversities in wasting of the bagasse, which emerges from the mill station after juice extraction. Almost all of the companies that produce raw sugar from cane sugar exploit some initiatives to gain from their waste in downstream industries including ethanol production, paper production, cattle feed and fuel, bioelectricity generation, and bioplastic production (11, 12).
In recent years, other new applications have been suggested for the bagasse, one of them is wastewater treatment (13-18). To date, removal of heavy metals, especially lead ions by means of bagasse, as a potentially effective adsorbent, has been developed (19-21). Various functional groups such as amino, hydroxyl, carboxyl, sulfate, and phosphate can be found in the chemical structure of bagasse, which may aid to adsorb heavy metals onto its surface (22). However, the feasibility of bagasse for water treatment is not satisfactory, and modification of its surface seems to be inevitable.
In this study, a simple SPE method was developed using bagasse modified with dithizone as a selective ligand that forms a strong complex with Pb (II). Dithizone contains N donor atoms, which are very specific for transition metal ions like Pb (23).
Batch process was performed to realize the adsorption properties of modified adsorbent. Some effective factors such as contact time, pH, volume of sample solution, and ionic strength effect were optimized.
2. Objectives
This study aimed to evaluate the adsorption of Pb (II) from water samples by bagasse modified with dithizone and determine the effect of different parameters involved in the process pH, contact time, volume of sample solution and electrolyte concentration, and finally to determine the appropriate adsorption isotherm.
3. Methods
3.1. Instrumental
An atomic absorption spectrophotometer (Varian AA240FS, Australia) equipped with a Photron Pb hallow cathode lamp (Australia) at the wavelength 283.3 nm was used for the determination of lead ions in solution samples. Structural analysis of dithizone-modified bagasse adsorbent was performed by X-ray diffractometer (XRD, Brucker D8 Discover, Germany). Infrared spectrums were obtained using a FTIR Spectrometer (Rayleigh WQF-510, BRAIC, China) to identify the chemical bonding and functional groups of the coated adsorbent. A Shaker (KS 130, IKA Works Basic Model, Germany) and a pH meter (Metrohm, 827, Switzerland) were used during the experiments.
3.2. Chemicals
The solutions were prepared by double distilled water with resistance more than 0.5 MΩ. All the chemicals and reagents used were of the analytical grade. Diphenylthiocarbazone (Dithizone, Dz), alkyldimethylbenzylammonium chloride (Benzalkonium chloride, ADBAC), potassium hydroxide granular, sodium hydroxide granular, hydrochloric acid (37% w/w), and nitric acid (65 % w/w) were purchased from Merck (Darmstadt, Germany). Bagasse was collected from a cane sugar factory located in southern Ahvaz (Iran). Analytical solutions of lead ions were prepared by stepwise dilution of 1000 mg L-1 standard solution (Romil, UK) with water.
3.3. Preparation of Dithizone-Modified Bagasse Adsorbent
Preparation and modification of bagasse with dithizone was performed according to experiments obtained in previous studies (24-26). Accordingly, bagasse was washed in running water for 24 hours to eliminate mud sticking to the trashes and remaining sugars in its structure. The sugar-out bagasse was dried in an oven at 105°C for 3 hours. The dried bagasse was grinded with a laboratory mill to pass through a 70-mesh sieve (The size of final particles were less than 0.21 mm). A total of 30 g fine particles of bagasse was poured in 500 mL KOH solution (10 %) and stirred for one night. Addition of KOH solution to bagasse particles facilitated the elimination of colorants, organic acids, lipids and proteins in the cellulosic structure of bagasse, and mostly important alkalized the surface of particles (27, 28). The alkalized particles were then washed with sufficient water until pH 7 in the eluate solution was achieved. To enhance the adsorptive properties of bagasse particles (BPs), dithizone was applied. As regards, the surface of the BPs is not proper for direct modification with Dz and pretreatment of the surface is imperative before modification. For this purpose, alkalized bagasse particles (BPs) were poured in 200 mL ADBAC solution (3 gL-1 in water) and stirred at 200 rpm for one night, followed by washing sufficiently with water to clean up the remainder of alkyldimethylbenzylammonium chloride (ADBAC) in the sphere of bagasse particles (BPs) At the end, the ADBAC-modified BPs were mixed with 200 mL Dz solution (1.5 g L-1 in ethanol) and stirred at 200 rpm for one night to complete the functionalization process on modified BPs. The modified bagasse particles Dithizone modified bagasse particles (Dz@BPs) were washed with ethanol solution (50%) several times and subsequently with water. The prepared adsorbent was held in distilled water prior to use.
3.4. Adsorption Procedure
The removal of Pb2+ onto Dz@BPs was studied in a batch process. Adsorption experiments were done on a shaker at 200 rpm using 250 mL conical flask. For this purpose, 50 mg Dz@BPs was added to 50 mL lead ions solution (10 mg L-1) at 25°C and pH 6.0. After 10 minutes, lead ions were adsorbed by Dz@BPs. Thereafter, the test solution was passed through Whatmann no. 41 filter paper and the remaining Pb (II) was determined by Varian FAAS.
3.5. Adsorption Study
The adsorption experiments of Pb2+ were studied at batch mode in the range of 20 - 200 mg L-1. A total of 50 mg adsorbent was added to 50 mL of metal ions solution in conical flask (pH 6.0, 25°C, speed of stirring 200 rpm). After 30 minutes of stirring, the Dz@BPs was separated from the solution with filter paper and the initial and final metal ions concentrations were determined. As required, several steps of dilution were performed on the samples to fix the results in the linear range of calibration curve.
3.6. Sampling
The proposed method was handled on two different water samples collected from the (1) Karoon river (Ahvaz, Iran) water and (2) waste water of a sugar factory (southern Ahvaz, Iran). Physicochemical properties of water samples are presented in Table 1. All the samples were passed through a 0.45 µm cellulose acetate membrane filter and preserved at pH 1.0 with HNO3 0.5 M. Prior to the analysis; pH of the samples was adjusted at 6.0 by NaOH 0.5 M.
| Sample Source | ||
|---|---|---|
| Physicochemical Property | Waste Water of Sugar Factory | Karoon River |
| EC, µS cm-1 | 2800 | 2500 |
| pH | 6.4 | 7.8 |
| Na+, mgL-1 | 212 | 151 |
| K+, mgL-1 | 23.5 | 20.2 |
| Ca2+, mgL-1 | 235.1 | 200.2 |
| Mg2+, mgL-1 | 43.5 | 46.4 |
| Mn2+, mgL-1 | 0.59 | 0.32 |
| Ni2+, mgL-1 | 0.10 | 0.05 |
| Zn2+, mgL-1 | 4.8 | 3.5 |
| Fe2+, mgL-1 | 1.5 | 0.5 |
| Al3+, mgL-1 | 0.16 | 0.12 |
| Cl-, mgL-1 | 190.4 | 149.1 |
| SO42-, mgL-1 | 305 | 238 |
| NO3-, mgL-1 | 46 | 33 |
| NO2-, mgL-1 | 1.2 | 0.9 |
4. Results
4.1. Characterization of the Dz@BPs
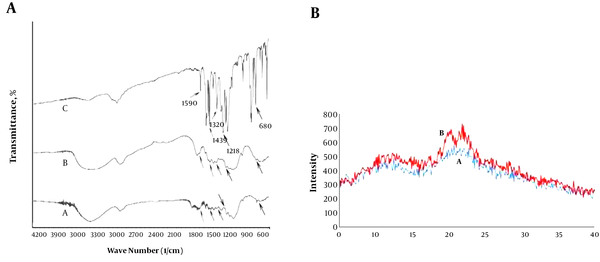
IR spectrum Figure 1A shows the characteristic peaks of uncoated bagasse particles (A) and dithizone-coated bagasse particles (B). Accordingly, the spectrum belongs to Dz@BPs Figure 1AB contains obvious peaks at 680 (C - H, phenyl group), 1,218 (-NCS-), 1,320 (N-phenyl), 1,439 (N - H, bending), and 1,590 cm-1 (C = C, aromatic) that are not available in the spectrum of uncoated bagasse particles Figure 1A. Appearance of these peaks is good evidence on adhesion of dithizone onto BPs. Figure 1B shows the variation on the XRD patterns of BPs after treatment with KOH and modification with ADBAC and dithizone. As shown, two special peaks related to cellulose at 20.1° and 22.4° appeared. An increase in crystallinity of Dz@BPs, with respect to BPs, was observed, which indicated that the hydrolysis was effective (29).
A, FTIR spectrums of PB (A), Dz@BPs (B) and Dz (C); B, X-ray diffraction pattern of bagasse particles (A) and dithizone-modified bagasse particles (B).
4.2. Effect of pH
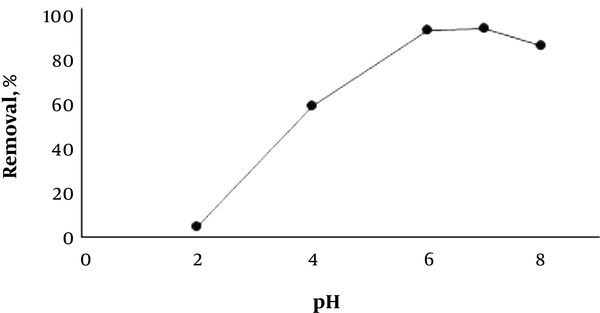
The effect of pH on the adsorption of Pb(II) by Dz@BPs was investigated at pH ranging from 2.0 - 8.0 (50 mL, Pb2+ 10 mg L-1, 50 mg adsorbent, 25°C, contact time 10 minutes, 200 rpm). According to the obtained results Figure 2, the best removal efficiency of Pb2+ was at pH 6.0 - 7.0 (> 99.5%). Variation of pH has a significant effect on the adsorption process. Increasing pH of solution accelerates the precipitation rate of lead ions with OH- due to the formation of Pb(OH)2 (Ksppb(OH)2 = 4.0 × 10-15 In addition, the feasibility of H+ to attract on dithizone molecules competes the adsorption process of Pb2+ on Dz@BPs.
The effect of pH of the solution for quantitative removal of Pb ions using Dz@BPs at 25°C
4.3. Effect of Contact Time
The contact time required to adsorb Pb (II) in sample solutions was studied at different contact times in the range of 0.5 - 30.0 minutes (10 mg L-1, 50 mL, 50 mg adsorbent, pH 6.0, 25°C, 200 rpm). After ending the removal process, test solutions were collected and analyzed using FAAS. According to the results, the time required to adsorb Pb (II) was quantitatively 1.0 minute (> 99 %). It is believed that the short time needed to adsorb lead ions is due to the high propensity of dithizone to make complex with Pb (II).
4.4. Effect of the Adsorbent Amount
The amount of Dz@BPs for removal of Pb ions from 50 mL sample solution (10 mg L-1) was studied in the range of 10 - 100 mg (pH 6.0, 25°C and 200 rpm). Acceptable adsorption efficiency (≥ 99%) was achieved when using 50 mg of Dz@BPs.
4.5. Adsorption Isotherm
To study the adsorption behavior of Pb (II) on Dz@BPs, Langmuir (representative of monolayer adsorption on homogenous surface) and Freundlich (representative of multilayer adsorption on heterogeneous surfaces) adsorption isotherm models were studied.
The capacity of Dz@BPs for adsorption of Pb (II) was determined by evaluating the difference between initial and final concentration of the solutions at batch mode (50 mL, contact time 60 min, pH 6.0, 25°C and stirring speed 200 rpm). Different concentrations of Pb (II) ranging from 20 to 200 mg L-1 in test solutions were examined with fixed amount of adsorbent (50 mg). The results Figure 3 showed that Freundlich model (R2 = 0.998) fitted well with respect to Langmuir (R2 = 0.985) model. The Freundlich isotherm equation was applied for explaining the correlation between the amount of adsorbed analyte and its equilibrium concentration in solution:
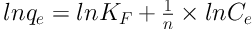
The plot of qe (mgg-1) versus Ce (mgL-1) for removal of Pb (II) by Dz@BPs at 25°C and pH 6
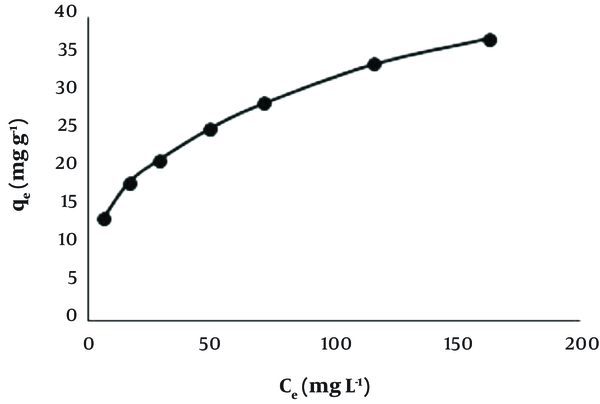
Where qe and Ce are the equilibrium adsorption capacity (mg g-1) and equilibrium heterogeneous multilayer concentration (mg L-1), respectively. KF and n are Freundlich constants. The linear relationship between ln qe and ln Ce (ln qe = 0.3099 ln Ce + 2.0341) represents the performance of the Freundlich model for the interpretation of adsorption of Pb ions on Dz@BPs. The experiments eventuated 7.64 and 3.22 for KF and n, respectively. Figure 3 Showed The plot of qe (mg g-1) versus Ce (mg L-1) for removal of Pb(II) by Dz@BPs at 25 ºC and pH 6.
4.6. Effect of Ionic Strength
According to previous knowledge, ionic strength may have a significant effect on the adsorption properties of an adsorbent. To investigate the effect of ionic strength (adjusted by 0.005 - 0.1 M KNO3) on lead adsorption by 50 mg Dz@BPs, 50 mL of sample solution (Pb2+ 10 mg L-1) at 25°C and pH 6.0 was selected. It was observed that the removal efficiency of lead ions was quantitative (95 %) at the concentrations below than 0.01 M KNO3.
4.7. Loading Capacity of Adsorbent
The loading capacity of Dz@BPs was determined under defined conditions (adsorbent amount 50 mg, pH 6, 25°C, stirring speed 200 rpm) by batch method. The adsorbent was poured in a 50 mL solution containing 200 mg L-1 of Pb ions and stirred for 60 minutes. Removal percent and adsorbed amount of Pb was determined by FAAS measurement of the initial and final concentrations of sample solution. The loading capacity was determined to be 37.20 mg g-1.
4.8. Reusability
Reusability of an absorbent can be a measure for its competency in the removal of hazardous materials in the environment. This ability for the adsorption of lead ions was experimented on the Dz@BPs in controlled conditions (Pb2+ 10 mg L-1, 50 mL, pH 6.0, adsorbent amount 50 mg, stirring speed 200 rpm and contact time 30 minutes). Prior to each step, 20 mL of HNO3 1 M and sufficient water were used to elute adsorbent. The results showed that the adsorption process can be iterated for four times without a considerable loss in its efficiency (> 95%).
4.9. Effect of Solution Volume on Removal Efficiency
To achieve an acceptable volume of lead sample solution per adsorbent amount, various sample volumes in the range of 50 - 250 mL were studied (50 mg adsorbent amounts, pH 6.0, contact time 60 min., stirring speed 200 rpm and 25°C). Volume of samples was incresaed while the total wheight of Pb2+ in solutions remained fixed at 0.5 mg. The obtained results showed that the effective adsorption (> 90 %) of Pb2+ was applicable up to150 mL of solutions. At volumes higher than 150 mL, the analyte was not adsorbed effectively due to the decrease in probability of collision between the adsorbent and lead ions.
4.10. Simulated Samples
The proposed SPE method was applied to the removal of lead ions in aqueous sample solutions. To determine the reliability and usability of the method, several 50 mL Pb2+ (spiked 5 and 10 mg L-1) contaminated water samples were prepared. The adsorption process was carried out at optimized conditions and removal percent was evaluated using FAAS technique. For the mentioned spiked levels of Pb (5 and 10 mg L-1), removal efficiencies of 94.4 % and 90.9 % were obtained for Karoon river samples; while the removal efficiencies of waste water of sugar factory samples were 93.6% and 89.5%, respectively.
5. Discussion
A simple, inexpensive and environmental friendly SPE of Pb (II) from aqueous solution has been successfully studied with dithizone-modified bagasse particles. To ensure the applicability of modification process, bare bagasse and modified adsorbent was compared. The results showed that the adsorption efficiency with dithizone-modified adsorbent was > 99.5% whereas 0.5% was obtained for unmodified bagasse in the same conditions. The adsorption property can be described by the Freundlich isotherm. A relatively short time (1.0 minutes) is adequate to accomplish the adsorption process. Table 2 demonstrates the comparison between some parameters of the present method and previously reported methods and adsorbents. Proposed method shows a high ability for removal of Pb (II) from water samples with relatively sophisticated matrices. According to the obtained results Figure 2, the best removal efficiency of Pb2+ was at pH 6.0-7.0 (> 99.5 %). Variation of pH has significant.
Comparison of the Proposed Method with Some of the Reported Methods
| Adsorbent Type | Qmax, mgg-1 | Contact Time, min | pH | Removal, % | Ref. |
|---|---|---|---|---|---|
| Bagasse fly ash | 2.5 | 80 | 6.0 | 98 | (6) |
| Olive cake | 18.14 | 60 | 6.0 | 80.6 | (30) |
| MMSCB 2 | 500.0 | 10 | 5.5 | - | (31) |
| Sugarcane bagasse | 6.37 | 120 | 5.0 | ~100 | (19) |
| Sugarcane bagasse treated by H2SO4 | 7.30 | 120 | 5.0 | ~100 | (19) |
| Untreated Sugarcane bagasse | 21.28 | 90 | 6.0 | 99.47 | (32) |
| Lagenaria vulgaris shell | 33.21 | 40 | 5.0 | ~100 | (33) |
| Dz@BPs | 37.20 | 1 | 6 | > 99.5 | This |
Acknowledgements
References
-
1.
Chauhan G, Chauhan UK. Relationship between heavy metal and transfer factor from soil to vegetable collected from waste water irrigated area of rewa (mp) india. Int J Technic Res Applicat. 2014:2320-8163.
-
2.
Sarkar B. Heavy metals in the environment. CRC Press; 2002.
-
3.
Shibamoto T, Bjeldanes LF. Introduction to food toxicology. Academic press; 2009.
-
4.
Kong J, Yue Q, Sun S, Gao B, Kan Y, Li Q, et al. Adsorption of Pb(II) from aqueous solution using keratin waste – hide waste: Equilibrium, kinetic and thermodynamic modeling studies. Chem Engin J. 2014;241:393-400. https://doi.org/10.1016/j.cej.2013.10.070.
-
5.
Qu X, Brame J, Li Q, Alvarez PJ. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc Chem Res. 2013;46(3):834-43. [PubMed ID: 22738389]. https://doi.org/10.1021/ar300029v.
-
6.
Gupta VK, Ali I. Removal of lead and chromium from wastewater using bagasse fly ash--a sugar industry waste. J Colloid Interface Sci. 2004;271(2):321-8. [PubMed ID: 14972608]. https://doi.org/10.1016/j.jcis.2003.11.007.
-
7.
Hamza IAA, Martincigh BS, Ngila JC, Nyamori VO. Adsorption studies of aqueous Pb(II) onto a sugarcane bagasse/multi-walled carbon nanotube composite. Phys Chem Earth. 2013;66:157-66. https://doi.org/10.1016/j.pce.2013.08.006.
-
8.
Pereira FV, Gurgel LV, Gil LF. Removal of Zn2+ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA dianhydride (EDTAD). J Hazard Mater. 2010;176(1-3):856-63. [PubMed ID: 20047793]. https://doi.org/10.1016/j.jhazmat.2009.11.115.
-
9.
Ayaz S, Aktas O, Findik N, Akca L. Phosphorus removal and effect of adsorbent type in a constructed wetland system. Desalin Water Treat. 2012;37(1-3):152-9. https://doi.org/10.1080/19443994.2012.661267.
-
10.
Ali I. New generation adsorbents for water treatment. Chem Rev. 2012;112(10):5073-91. [PubMed ID: 22731247]. https://doi.org/10.1021/cr300133d.
-
11.
Ding W, Dong X, Ime IM, Gao B, Ma LQ. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere. 2014;105:68-74. [PubMed ID: 24393563]. https://doi.org/10.1016/j.chemosphere.2013.12.042.
-
12.
Kulkarni DP. Cane sugar manufacture in India. Digambar Purushottam; 1996.
-
13.
Mukherjee S, Kumar S, Misra AK, Fan M. Removal of phenols from water environment by activated carbon, bagasse ash and wood charcoal. Chem Engin J. 2007;129(1-3):133-42. https://doi.org/10.1016/j.cej.2006.10.030.
-
14.
Rao M, Parwate AV, Bhole AG. Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Manag. 2002;22(7):821-30. https://doi.org/10.1016/s0956-053x(02)00011-9.
-
15.
McKay G, El Geundi M, Nassar MM. Equilibrium studies during the removal of dyestuffs from aqueous solutions using Bagasse pith. Water Res. 1987;21(12):1513-20. https://doi.org/10.1016/0043-1354(87)90135-7.
-
16.
Zaheer S, Bhatti HN, Sdaf S, Safa Y, Zia-ur-Ruhman M. Biosorptioncharateristics of sugarcane bagasse for the removal of foron blue E-BL bye from aqueous solutions, The J. Animal Plants Sci. 2014;24(1):272-9.
-
17.
Mukherjee K, Saha R, Ghosh A, Ghosh SK, Maji PK, Saha B. Surfactant-assisted bioremediation of hexavalent chromium by use of an aqueous extract of sugarcane bagasse. Res Chem Intermediates. 2013;40(4):1727-34. https://doi.org/10.1007/s11164-013-1077-4.
-
18.
Azhar SS, Liew AG, Suhardy D, Hafiz KF, Hatim MDI. Dye Removal from Aqueous Solution by using Adsorption on Treated Sugarcane Bagasse. Am J Appl Sci. 2005;2(11):1499-503. https://doi.org/10.3844/ajassp.2005.1499.1503.
-
19.
Martin-Lara MA, Rico ILR, Vicente ICA, Garcia GB, de Hoces MC. Modification of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions. Desalination. 2010;256(1-3):58-63. https://doi.org/10.1016/j.desal.2010.02.015.
-
20.
Karnitz O Jr, Gurgel LV, de Melo JC, Botaro VR, Melo TM, de Freitas Gil RP, et al. Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresour Technol. 2007;98(6):1291-7. [PubMed ID: 16843656]. https://doi.org/10.1016/j.biortech.2006.05.013.
-
21.
Rezende CA, de Lima MA, Maziero P, deAzevedo ER, Garcia W, Polikarpov I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels. 2011;4(1):54. [PubMed ID: 22122978]. [PubMed Central ID: PMC3377919]. https://doi.org/10.1186/1754-6834-4-54.
-
22.
Sud D, Mahajan G, Kaur MP. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions - a review. Bioresour Technol. 2008;99(14):6017-27. [PubMed ID: 18280151]. https://doi.org/10.1016/j.biortech.2007.11.064.
-
23.
Siswanta D, Ola PD. Adsorption Characteristics of Pb(II) and Cd(II) Ions on Dithizone-loaded Natural Zeolite. J Ion Exchange. 2007;18(4):564-9. https://doi.org/10.5182/jaie.18.564.
-
24.
Zuluaga R, Putaux JL, Cruz J, Vélez J, Mondragon I, Gañán P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydrate Polymers. 2009;76(1):51-9. https://doi.org/10.1016/j.carbpol.2008.09.024.
-
25.
Kästner U, Hoffmann H, Dönges R, Ehrler R. Interactions between modified hydroxyethyl cellulose (HEC) and surfactants. Colloids Surfaces Physicochem Engin Aspects. 1996;112(2-3):209-25. https://doi.org/10.1016/0927-7757(96)03557-1.
-
26.
Syverud K, Xhanari K, Chinga-Carrasco G, Yu Y, Stenius P. Films made of cellulose nanofibrils: surface modification by adsorption of a cationic surfactant and characterization by computer-assisted electron microscopy. J Nanoparticle Res. 2010;13(2):773-82. https://doi.org/10.1007/s11051-010-0077-1.
-
27.
Harris CI. Method of extracting protein and lipid from raw vegetable materials. United States patent; 1963.
-
28.
Miller KB, Hurst WJ, Payne MJ, Stuart DA, Apgar J, Sweigart DS, et al. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J Agric Food Chem. 2008;56(18):8527-33. [PubMed ID: 18710243]. https://doi.org/10.1021/jf801670p.
-
29.
Teixeira EM, Bondancia TJ, Teodoro KBR, Correa AC, Marconcini JM, Mattoso LHC. Sugarcane bagasse whiskers: Extraction and characterizations. Indust Crops Prod. 2011;33(1):63-6. https://doi.org/10.1016/j.indcrop.2010.08.009.
-
30.
Doyurum S, Celik A. Pb(II) and Cd(II) removal from aqueous solutions by olive cake. J Hazard Mater. 2006;138(1):22-8. [PubMed ID: 16806680]. https://doi.org/10.1016/j.jhazmat.2006.03.071.
-
31.
Gurgel LVA, Freitas RP, Gil LF. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by sugarcane bagasse and mercerized sugarcane bagasse chemically modified with succinic anhydride. Carbohydrate Polymers. 2008;74(4):922-9. https://doi.org/10.1016/j.carbpol.2008.05.023.
-
32.
Pranata Putra W, Kamari A, Najiah Mohd Yusoff S, Fauziah Ishak C, Mohamed A, Hashim N, et al. Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies. J Encapsul Adsorpt Sci. 2014;4(1):25-35. https://doi.org/10.4236/jeas.2014.41004.
-
33.
Kostic M, Radovic M, Mitrovic J, Antonijevic M, Bojic D, Petrovic M, et al. Using xanthated Lagenaria vulgaris shell biosorbent for removal of Pb(II) ions from wastewater. J Iran Chem Soc. 2013;11(2):565-78. https://doi.org/10.1007/s13738-013-0326-1.
