Abstract
Background:
As known, there is a high correlation between Helicobacter pylori infection and gastric carcinoma.Objectives:
Concerning the important role of adhesin HpaA of H. pylori in the infection process, we aimed to explore whether HpaA promotes gastric cancer metastasis.Methods:
In this study, the levels of IL-21, MMP-2, and MMP-9 in patients’ biopsies with H. pylori infection were compared with post-treatment condition. The levels of IL-21 from CD4+ T cells and culture supernatants with the recombinant HpaA treatment were detected, and then the levels of MMP-2, MMP-9, and metastasis were detected and verified via AGS cells co-cultured with aforesaid CD4+ T cells.Results:
Our results showed that higher levels of IL-21, MMP-2, and MMP-9 in patients’ biopsies with H. pylori infection than without H. pylori infection. Adhesin HpaA induced more IL-21 via CD4+ T cells, and IL-21 induced high MMP-2 and MMP-9 via AGS cells. In particular, HpaA caused this serial reaction to improve the migration of AGS cells, and aptamer HA6 (our previous report) and anti-IL-21 mcAb reduced the above phenomenon remarkably.Conclusions:
In summary, our research suggested that adhesin HpaA plays a significant role in the process of gastric carcinoma cell metastasis via IL-21 from HpaA-induced T cells, and aptamer HA6 may be a potential therapeutic agent for H. pylori treatment.Keywords
Helicobacter pylori Adhesin HpaA Interleukin-21 Matrix Metalloproteinase Migration
1. Background
Helicobacter pylori is a bacterium living in the stomach of over 50% of the population. Its infection may result in gastroduodenal disorders, including chronic active gastritis, peptic ulcer, Mucosa-associated Lymphoid Tissue (MALT) lymphoma, and even gastric adenocarcinoma (1-3). The mechanism through which H. pylori damages the host is complex. For the past several decades, evidence suggested that adherence to the gastric epithelium and persistent colonization by H. pylori were the critical parameters for the development of gastric malignancies (4). Helicobacter pylori has developed a set of virulence factors on its outer membrane, called adhesins, such as HpaA, BabA, SabA, AlpA/B, OipA, and HopZ. They can recognize and bind to receptors on epithelial cells of the gastric mucosa to facilitate H. pylori to establish a successful infection (5-8). Among them, neuraminyllactose-binding hemagglutinin (HpaA), a highly conserved lipoprotein (9), regulates the colonization and other different stages of the pathogenic process of H. pylori, and is expected to be a target for H. pylori treatment (10).
Interleukin-21 (IL-21) is produced by CD4+ T cells and modulates a variety of immune cells (11). It maintains chronic inflammation and/or damages the tissues by facilitating the recruitment of immune cells (12), expanding autoreactive T cells (13), and producing extracellular matrix metalloproteinases (14). A previous study reported higher levels of IL-21 in the gastric mucosa after H. pylori infection to be involved in H. pylori pathogenesis (15). However, it remains unknown whether adhesin HpaA of H. pylori intensifies the inflammatory response, metastasis of gastric cancer, and injury in the gastric mucosa by IL-21. In our previous study, a higher expression of IL-21 in the gastric mucosa of patients with H. pylori infection was examined and compared with the condition after H. pylori radical cure (16). We then analyzed the impact of adhesin HpaA on the IL-21 expression levels in AGS cells. Furthermore, in our previous study, ssDNA aptamer HA6 could bind to and block the adherence domain of HpaA, in particular. Thus, it was introduced in this study to prove whether HpaA could induce IL-21 secretion from T cells.
2. Objectives
Given the important role of adhesin HpaA from H. pylori in its invasion and pathopoiesia, we aimed to explore how to intensify the inflammatory response and mucosal injury by IL-21 induced from -T cells by adhesin HpaA of H. pylori. Particularly, AGS cells (gastric cancer cell lines) were used to examine whether IL-21 promotes the migration of AGS.
3. Methods
3.1. Materials
Helicobacter pylori ATCC26695 standard strain was donated by Professor Shao Shihe from Jiangsu University. The pET28a plasmid was kept in our laboratory (The Basic Medical Laboratory, The Second Affiliated Hospital of Wannan Medical College, Anhui Wuhu, China). The Bacterial Genomic DNA Preparation Kit was purchased from TianGen Co., Ltd. (Beijing, China). The RNA Extraction Kit and IPTG (isopropyl-β-d-thiogalactoside) were obtained from Solarbio Co., Ltd. (Shanghai, China). The superscript first-strand cDNA synthesis kit, restriction enzyme BamH I, and Hind III and T4 ligase were acquired from Fermentas (Finland). Primers were produced by Sangon Biotech Co., Ltd. (Shanghai China). The human IL-21 ELISA kit, Polyclonal-mouse-reactive anti-MMP-2, anti-MMP-9, anti-IL-21, and anti-β-actin were obtained from Abcam Biotechnology (UK).
Columbia Agar and sterile defibrinated sheep blood were acquired from Hopebio Co., Ltd. (Qingdao, China). Fetal Bovine Serum (FBS) was acquired from Gibco (Grand Island, N.Y., USA). Micro-aerobic AnaeroPack was acquired from MGC (Japan). The DNA marker and protein marker were acquired from LabLead Co., Ltd. (Beijing, China). Kanamycin, gentamicin, and salmon sperm DNA were acquired from Sigma-Aldrich (St. Louis, MO, USA). The PCR mix was acquired from Thermo Fisher (Germany). The Ni-NAT column was acquired from Novagen (Malaysia).
3.2. Patients’ Specimens
Specimens of gastric mucosa were acquired from 56 patients (28 males, 28 females; mean age: 40.2 ± 13.32 years) from February 2017 to December 2018 at the Digestive Endoscopy Center (The Second Affiliated Hospital of Wannan Medical College, Wuhu city, Anhui province, China). The selected patients had symptoms of gastritis or gastric ulcers and were H. pylori-positive, but had no other gastric lesions. They were examined for their gastric mucosa before and after treatment. Not fewer than two pieces of specimens were gained from each patient to analyze by a gastroscope. Each gastric mucosa was detected by the rapid urease test. In our study, the donors were selected according to the standard, H.pylori positive before treatment, and H.pylori negative after treatment. Then, the 112 pieces of gastric mucosa samples were categorized into two groups: the H. pylori-positive group and the corresponding H. pylori- eradication group. None of the H. pylori-positive patients had undergone treatment with non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics, or proton pump inhibitors at least one-month before testing. Quadruple therapy was used to eradicate H. pylori. The infection status was analyzed using the rapid urease test, pathological staining, 14C Urea breath tests, and H. pylori-specific ureC PCR. The two or more positive results in the above four methods for each donor confirmed the H. pylori infection. The samples were frozen at -80°C immediately after collection for future use.
3.3. Helicobacter pylori Culture
Helicobacter pylori standard strain 26695 was cultured in Columbia Agar plates supplemented with 5% FBS under microaerophilic conditions (5% O2, 10% CO2, and 85% N2, 37°C) for 48 to 72 h. Helicobacter pylori was collected at the logarithmic phase and used for future experiments.
3.4. Cell Culture
The AGS-I cells were maintained in our laboratory (The Basic Medical Laboratory, The Second Affiliated Hospital of Wannan Medical College). The cells were maintained in RPMI-1640 medium containing 10% FBS in a humidified incubator (5% CO2, 37°C).
3.5. Reverse Transcription and Real-time PCR
The total RNA was extracted from the gastric biopsies using the total RNA extraction kit (Cat#1218325, ThermoFisher) and reverse-transcribed into cDNA using the superscript first-strand cDNA synthesis kit based on the manufacturers’ specifications. The primer sequences are listed in Table 1. The RT-PCR was done in 20-μl volumes containing 2 μl of synthesized cDNA solution, 10 μl of 2×PCR Master Mix, 500 nM of each primer, and sterile water added to 20 μl on the ABI Step One Real-Time PCR System (Applied Biosystems). The thermal cycle was set as follows: 95°C for 5 min, 40 cycles of 95°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Each assay was performed in duplicate, and the relative gene expression was normalized to β-actin following calculations by the 2-ΔΔCt method.
The Primer Sequences of IL-21, MMP-2, and MMP-9 for qRT-PCR
3.6. Construction of Expression Plasmid
Primers designed to amplify the HapA sequences from H. pylori standard strain 26695 were as follows: F: 5'-CGGATCCTGCAGCCCGCATATTATTG-3' and R: 5'-CAAGCTTTCGGTTTCTTTTGCCTTTT-3'. The PCR setting condition was 95°C for 5 min, 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, ×30; and then 72°C for 5 min, ×1. The resultant DNA sequence was inserted into the BamH I and Hind III sites of the pET28a vector to construct the pET28a-HpaA recombinant expression plasmid. The HapA sequences of the recombinant vector were confirmed by sequencing.
3.7. Expression and Purification of Recombinant HapA Fusion Protein
Escherichia coli strain BL21 (DE3) was transformed with pET28a-HpaA and named pET28a-HpaA-BL21. Then, pET28a-HpaA-BL21 was grown in LB medium and induced using IPTG (1.0 mmol/l) at 37°C. The bacteria were harvested at 1, 2, 3, and 4 h, and the total protein was collected through centrifugation after the bacteria were lysed with lysozyme. Protein expression was analyzed by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), followed by visualization of protein bands by Coomassie staining. Subsequently, the recombinant HapA fusion protein was purified by the Ni-nitrilotriacetic acid resin and verified by SDS-PAGE (17). The recombinant HpaA was analyzed by peptide mass fingerprinting again.
3.8. CD4+T-Cell Immunopurification
Ficoll-Hypaque gradient sedimentation was conducted to isolate Peripheral Blood Mononuclear Cells (PBMCs). Then, CD4+T-lymphocytes were selected positively. Briefly, PBMCs were incubated with monoclonal antibodies against CD4 and then with anti-murine IgG conjugated-magnetic beads (Dynabeads M-450, Dynal, Oslo, Norway) for 30 min at 4°C (18). Selected CD4+ T cells were re-suspended using RPMI-1640 media supplemented with 10% FBS and grown in a humidified incubator (5% CO2, 37°C). The purity was always ≥ 90% based on the flow cytometry analysis (data not shown).
3.9. HpaA Stimulation of IL-21 Secretion from CD4+ T Cells
The ssDNA aptamer, specific binding to adhesin HpaA, was screened by SELEX. In our previous study, the ssDNA aptamer could bound to and block the adherence domain of HpaA, in particular (19). This aptamer was used to verify whether HpaA stimulates the IL-21 secretion from CD4+ T cells.
3.10. Western Blotting
Cells were lysed in RIPA buffer containing 1×Phenylmethanesulfonyl fluoride (PMSF), followed by protein concentration measurement by an ND-1000 spectrophotometer. Next, 100 μg protein was resolved by the 10% SDS-PAGE gel and subsequently electrotransferred to PVDF membranes. After blocking with 5% non-fat milk in 1×TBST for 2 h at Room Temperature (RT), primary antibodies against IL-21, MMP-2, MMP-9, and β-actin were applied for overnight incubation at 4°C. Following five times of 1×TBST washes, the membrane was incubated with HRP-conjugated goat anti-mouse IgG antibody for one hour at RT. Finally, protein bands were visualized by an Enhanced Chemiluminescence (ECL) system (Image Quant LAS 4000 mini, Pittsburgh, PA, USA). The relative band densities were measured using ImageJ (https://imagej.nih.gov/ij/).
3.11. ELISA Analysis of Cytokines
For the ELISA assay, 6×105 CD4+ T cells were grown in 12-well plates until reaching 70% confluence. The cells were co-cultured with the HapA fusion protein (10 μg/mL) and H. pylori (at a Multiplicity of Infection (MOI) = 100:1 (H. pylori: AGS cells)), in sequence, for 24 h. Next, the cell culture supernatant was analyzed by ELISA according to the manufacturer’s specifications. All samples were run in triplicate and the mean absorbance was calculated.
3.12. Trans-Well Invasion Experiment
To explore the migration of AGS cells via IL-21 from HpaA-induced CD4+ T cells, we designed a Trans-well invasion experiment to observed the AGS cells' metastasis. For this purpose, 2 × 104 AGS cells were cultured in the upper culture wells of a 96-well plate (Code: 3374, Corning, USA) while the lower culture wells were loaded with different treatments. According to the content of lower culture wells, there were seven groups, including the control group (culture medium), L-21 treatment group (1 ng/mL IL-21), IL-21+anti-IL-21 mcAb treatment group (1 ng/mL IL-21 and 10 ng/mL anti-IL-21 mcAb), HpaA+T cells treatment group (10 ng/mL HpaA-induced 1×104 CD4+T cells), HpaA+T cells+anti-IL-21 mcAb treatment group (10 μg/mL HpaA-induced 1×104 CD4+T cells and 10 ng/mL anti-IL-21 mcAb), HpaA+Aptamer HA6+T cells treatment group (10 μg/mL HpaA with 1 nM Aptamer HA6 and 1×104 CD4+T cells), and Aptamer HA6+T cells treatment group (1 nM Aptamer HA6 and 1×104 CD4+T cells). Then, AGS cells were counted on the bottom of the upper wells for migration assessment.
3.13. Statistical Analysis
All data were expressed as mean±Standard Deviation (SD) based on three independent experiments. The statistical software SPSS 19.0 was used to analyze the data with Student’s t-test between every two comparable groups or one-way ANOVA between different groups. In the analyses, P < 0.05 was defined as statistically significant.
4. Results
4.1. Lower levels of IL-21, MMP-2, and MMP-9 in Gastric Mucosa with Helicobacter pylori Infection Compared to After Radication
To determine the expression levels of IL-21, MMP-2, and MMP-9 in biopsies of gastric mucosa from patients with H. pylori infection and radical cure, three targeted molecular species of gastric mucosa tissue samples from 56 patients, before and after H. pylori radical cure, were detected by Western blotting. The data of Western blotting showed that IL-21, MMP-2, and MMP-9 expressions of gastric mucosa form H. pylori radication patients decreased significantly compared to those in the infection stage (Figure 1). The results showed that the IL-21 protein level was lower in the H. pylori radication group than in the H. pylori-positive group (t=3.069, P=0.0107) (Figure 2A). Similar results were also found for MMP-2 and MMP-9 expressions (t=11.42, P < 0.001 and t=13.04, P < 0.001, respectively) (Figure 2B and C).
Detection and comparison of the levels of IL-21, MMP-2, and MMP-9 from 56 gastric mucosa tissue samples before and after Helicobacter pylori radical cure by Western blotting.
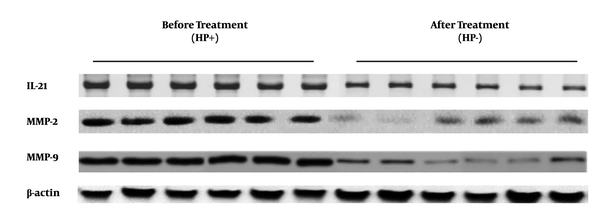
Detection and comparison of the protein levels of IL-21, MMP-2, and MMP-9 from gastric mucosa samples before and after Helicobacter pylori radical cure by Western blotting. **stand for P < 0.01.

4.2. IL-21, MMP-2, and MMP-9 Expressions of mRNA Were Lower in Gastric Mucosa Tissues with Helicobacter pylori Radication Compared to Before Treatment
Consistent with previous Western blotting results, the RT-PCR analysis of 56 gastric mucosa tissues before and after H. pylori radical cure showed a reduction in IL-21, MMP-2, and MMP-9 expressions in the radiation group than in the H. pylori-positive group (Figure 3). The RT-PCR results only showed a part of all figures. Collectively, these data suggested that IL-21, MMP-2, and MMP-9 expressions were reduced at both transcript and protein levels in the H. pylori radication group compared to the H. pylori-positive group.
Detection and comparison of the mRNA levels of IL-21, MMP-2, and MMP-9 from gastric mucosa before and after the Helicobacter pylori radical cure by RT-PCR. **stand for P < 0.01.

4.3. Expression and Identification of HapA Fusion Protein
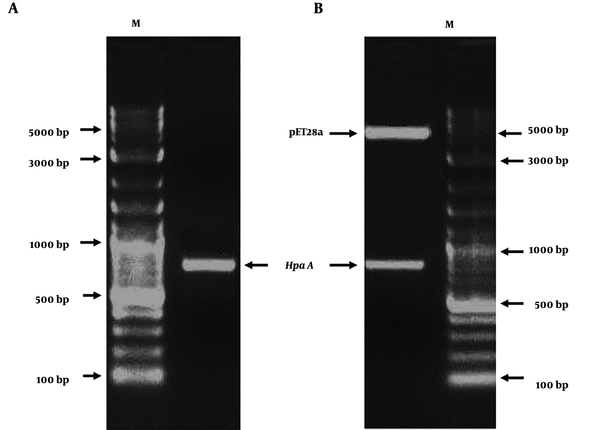
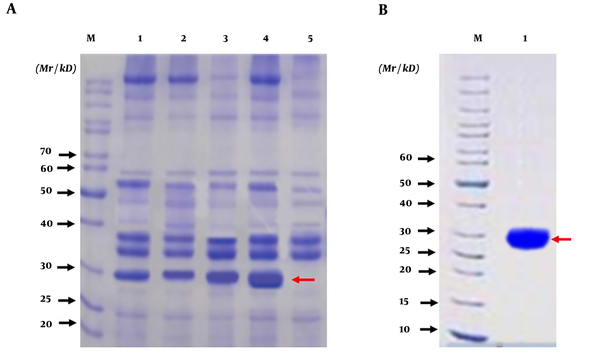
The HapA DNA fragment was amplified from H. pylori ATCC26695 by PCR. The PCR product was approximately 699 bp (Figure 4A). The amplified HapA DNA fragment was inserted into the BamHI and HindIII sites of the pET28a vector to construct the expression vector pET28a/HpaA and verified by digestion by restriction enzymes (BamHI and HindIII) (Figure 4B). Then, pET28a-HpaA was transformed into an E. coli strain to express the HpaA fusion protein. The recombinant protein was resolved by SDS-PAGE and showed an expected molecular weight of 30 kDa (Figure 5A). Next, the recombinant HapA proteins were purified using Ni–NTA affinity chromatography and verified through SDS-PAGE again (Figure 5B). The data of recombinant protein HapA were analyzed by peptide mass fingerprinting.
The construction and identification of recombinant expression vector pET28a/HpaA. (A) PCR-amplified HapA DNA sequences from Helicobacter pylori ATCC26695. (B) Identification of recombinant expression vector pET28a/HpaA by the restricted endonuclease enzyme. M represents the DNA marker.
The expression, purification, and identification of recombinant HapA protein. (A) Analysis of expressed recombinant protein HpaA from Escherichia coli DE3 (BL21) by SDS-PAGE; M: protein marker; Lanes 1, 2, 3, 4 are the lysates of induced E. coli DE3 with IPTG at 1 h, 2 h, 3 h, and 4 h, respectively; Lane 5 is the lysate of induced E. coli DE3 without IPTG; (B) Analysis of purified recombinant protein HpaA by SDS-PAGE; M: protein marker; Lane 1: purified recombinant protein HpaA (the recombinant protein HpaA is indicated by red arrows in figures)
4.4. HpaA Can Improve the IL-21 Release via Induced CD4+T Cells in Vitro
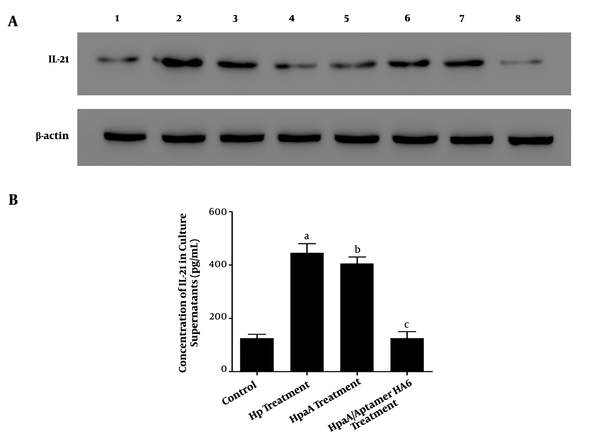
Our results showed that the IL-21 expression was greater in CD4+ T cells with H. pylori infection and biopsy specimens of H. pylori-positive patients than in CD4+ T cells without treatment (Figure 6A). Moreover, its expression was increased in CD4+ T cells with HapA fusion protein treatment in a time-dependent manner (Figure 6A). We also detected the release of IL-21 in CD4+ T cell culture supernatant with different treatments by ELISA. Our data showed higher IL-21 expression levels in the H. pylori infection group and HapA fusion protein treatment group than in CD4+ T cells without treatment (Figure 6B). However, the expression levels of IL-21 protein were not statistically significantly different between the H. pylori infection group and HapA fusion protein treatment group (Figure 6B).
The IL-21 levels of CD4+ T cells with different treatments. (A) Western blotting analysis of IL-21 expression in CD4+ T cells with different treatments; Lane 1: The levels of IL-21 and β-actin in CD4+T cells without treatment; Lane 2: The levels of IL-21 and β-actin in CD4+ T cells with Helicobacter. pylori treatment (CD4+ T co-cultured with H. pylori at MOI of 100 for 24 h); Lane 3: The IL-21 and β-actin levels of CD4+ T cells from gastric mucosa of patients with H. pylori infection; Lane 4 to lane 7: The IL-21 and β-actin levels of CD4+ T cells with recombinant HpaA protein (10 μg/mL) treatment at 3 h, 6 h, 12 h, and 24 h, respectively; Lane 8: The levels of IL-21 and β-actin in CD4+T cells with HpaA protein and aptamer HA6 co-treatment. (B) Detection and comparison of the concentration of IL-21 from CD4+T cells' culture supernatants with different treatments by ELISA; H. pylori treatment group: CD4+ T cells were co-cultured with H. pylori at an MOI of 100 for 24 h; HapA treatment group: CD4+ T cells were treated with HpaA protein (10 μg/mL) for 24 h; HapA/aptamer HA6 treatment group: CD4+ T cells were treated with HpaA protein (10 μg/mL) and aptamer HA6 (1 nM) for 24 h. a stands for Hp treatment groups vs. control group, P = 0.0176; b stands for HpaA treatment groups vs. control group, P = 0.0123; c stands for HpaA/Apatamer HA6 treatment groups vs. control group; P = 0.3639, no statistical significance.
4.5. The IL-21 Secretion of Adhesin HpaA-induced CD4+T-cells from Helicobacter pylori Promoted the Expression of MMP-2 and MMP-9 in AGS Cells
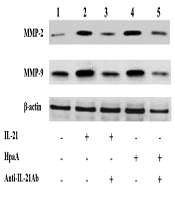
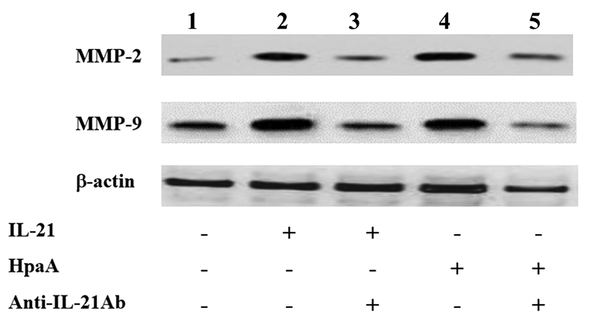
The T cell-derived cytokines were shown to induce MMP production, leading to mucosal ulceration and gastric cancer migration. Therefore, the IL-21 secretion of adhesin HpaA-stimulated CD4+ T cells from H. pylori regulated the MMP expression in AGS cells by Western blotting. The expression of MMP-2 and MMP-9 was increased in AGS cells with IL-21 treatment when compared to the control group (Figure 7). Subsequently, AGS cells were co-cultured with the adhesin HpaA-stimulated CD4+ T cells. The Western blotting results showed the MMP-2 and MMP-9 protein upregulation (Figure 7) while the pretreatment of AGS cells with adhesin HpaA-stimulated CD4+ T cells before adding the anti-IL-21 antibody downregulated the MMP-2 and MMP-9 expression (Figure 7). Altogether, the IL-21 secretion of adhesin HpaA-stimulated T cells from H. pylori increased the MMP-2 and MMP-9 expressions in AGS cells.
Western blotting analyses of MMP-2 and MMP-9 expressions in AGS cells with different treatments. Lane 1: AGS cells without treatment as the control; Lane 2: AGS cells treated with the IL-21 antibody (50 ng/ml) for 24 h; Lane 3: AGS cells pretreated with the IL-21 antibody (50 ng/ml) before adding the anti-IL-21 antibody (10 μg/ml) and then cultured for further 24 h; Lane 4: AGS cells co-cultured with adhesin HpaA (10 μg/mL)-stimulated CD4+ T cells for 24 h; Lane 5: AGS cells pre-co-cultured with adhesin HpaA (10 μg/mL)-stimulated CD4+ T cells before adding the anti-IL-21 antibody (10 μg/mL) and then cultured for further 24 h; The total proteins from AGS cells with different treatments were isolated for Western blotting analysis.
4.6. HpaA Promoted the Migration of AGS via IL-21 Secretion from CD4+T Cells by Trans-Well
To analyze the AGS cells’ migration with different treatments, AGS cells were cultured in the upper culture well, while the lower culture wells were loaded with treatment agents. The AGS cells were counted on the bottom of the upper well for migration assessment. The data showed more AGS cells in the IL-21 treatment group or HpaA-induced CD4+ T cells than in the control group (culture medium) on the bottom of the upper culture well. However, anti-IL-21 mcAb could decrease the AGS cells in the IL-21 treatment group remarkably. Similarly, The AGS cells migration was decreased when aptamer HA6 bonded HpaA to cause the level of IL-21 reducing from CD4+ T cells. The number of AGS cells migration was lower than the no aptamer HA6 treatment group. Although the number of AGS cells in the control group was lower than that in the IL-21+anti-IL-21 mcAb treatment group and HpaA+Aptamer HA6+CD4+ T cell treatment group, these differences were not statistically significant (Figure 8).
Analysis of AGS cell migration with different treatments via Trans-well. The AGS cells were cultured in upper culture wells but lower culture wells were loaded with treatments. The number of AGS cells was counted on the bottom of the upper wells for migration assessment. a stands for the IL-21 treatment group vs. the control group (culture medium), P = 0.0309; b stands for the IL-21 and anti-IL-21 mcAb co-treatment group vs. the control group, P = 0.8919; c stands for the CD4+ T cells with HpaA treatment group vs. the control group, P = 0.0365; d stands for the CD4+ T cells with HpaA and anti-IL-21 mcAb co-treatment group vs. the control group, P = 0.2280; e stands for CD4+ T cells with the HpaA and aptamer HA6 co-treatment group vs. the control group, P = 0.3118; f stands for the CD4+ T cells with aptamer HA6 co-treatment group vs. the control group, P = 0.1695.
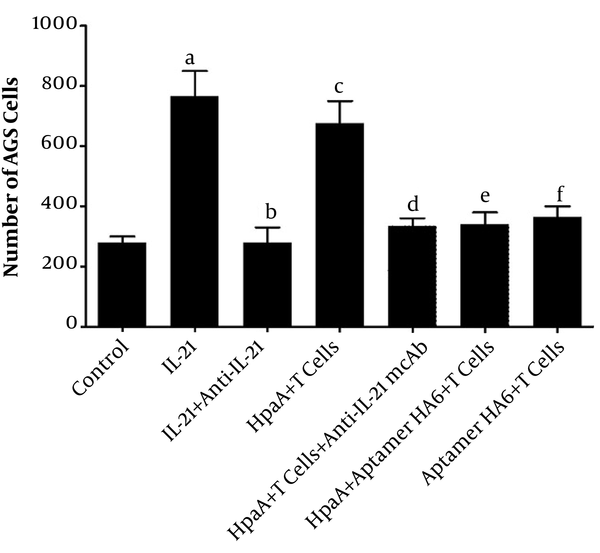
5. Discussion
In our previous study, we found an interesting phenomenon that IL-21, MMP-2, and MMP-9 were highly expressed in the gastric mucosa with H. pylori infection. To get the detail of the phenomenon, we examined some pieces of gastric mucosa specimens, and the results showed that the levels of the three mentioned indicators had a positive correlation with loading bacteria in the fresh gastric mucosa (data not shown). According to the above description, we suggested that the level of IL-21 elevated at the beginning of adherence with H. pylori. And then, five kinds of recombinant adhesin (HpaA, BabA, SabA, AlpA, and HopZ) were used for screening experiments before this research, and HpaA showed strong ability release IL-21 via stimulated CD4+ T cells remarkably. Thus, we focused on HpaA and conducted the current research based on a large number of preliminary experimental results.
In this research, adhesin HpaA from H. pylori ATCC 26695 was cloned and expressed to prove its role in the gastric mucosa with H. pylori infection. The long-term chronic inflammation caused by H. pylori has been suggested to lead to the adverse outcomes of colonization, such as chronic gastritis, intestinal metaplasia, and gastric cancer. Besides, IL-21 induces proliferation and increases cell viability and cytokine production in immune cells (20). Bagheri et al. have indicated the upregulation of IL-21 mRNA in the gastric mucosa upon H. pylori infection, which was positively correlated with the severity of chronic inflammation (15). Similar to previous reports (15), we also found that the IL-21 expression at both transcript and protein levels was enhanced in the gastric mucosa of H. pylori-infected patients and patients with H. pylori radical elimination. To determine the impact of adhesin HpaA of H. pylori on IL-21 production, CD4+ T cells were stimulated by the recombinant HpaA protein for different periods. The results showed that HpaA could improve the IL-21 release by induced CD4+ T cells in a time-dependent manner.
As known, MMPs are a group of neutral endopeptidases that play a key role in Extracellular Matrix (ECM) remodeling, inflammatory processes, and cell proliferation. Accumulated evidence has indicated that H. pylori-associated gastritis and gastric and duodenal ulcers are closely associated with cleaving and remodeling of the ECM by MMPs (21-24). Previous studies using patient biopsies have indicated a dramatic elevation in MMP-2 and MMP-9 expressions upon H. pylori infections (25, 26). In this study, similar to previous reports, we discovered lower MMP-2 and MMP-9 expressions in the H. pylori eradication group than in the H. pylori infection group.
Caruso et al. reported that IL-21 was increased in the H. pylori-positive gastric mucosa and AGS cells, which upregulated MMP-2 and MMP-9 (but not MMP-1, MMP-3, or MMP-7) expression, leading to mucosal ulceration and epithelial damage (14). Therefore, we developed a hypothesis that MMP-2 and MMP-9 expression may be regulated by the IL-21 secretion of adhesin HpaA-stimulated T cells from H. pylori. In subsequent experiments, AGS cells were co-cultured with adhesin HpaA-stimulated CD4+ T cells. As shown in Figure 4, MMP-2 and MMP-9 were upregulated in AGS cells co-cultured with adhesin HpaA-stimulated CD4+ T cells. However, the suppression of IL-21 led to the decreased production of MMP-2 and MMP-9 in AGS cells co-cultured with adhesin HpaA-stimulated CD4+ T cells. These results indicated that the IL-21 secretion of adhesin HpaA-stimulated CD4+ T cells from H. pylori increased the MMP-2 and MMP-9 expressions in AGS cells. Besides, the correlation between the migration of AGS cells and IL-21 was proven by trans-well invasion experiments, and anti-IL-21 mcAb reduced the number of AGS cells on the bottom of upper culture wells.
Indeed, HpaA and Aptamer HA6 were introduced to prove that IL-21 can promote AGS cells’ migration again. The data showed that aptamer HA6 could reduce AGS cells’ migration via blocking HpaA-stimulated CD4+ T cells. Other kinds of adhesins or virulent factors of H. pylori may cause similar or opposite functions via other pathways because many kinds of virulence factors from H.pylori play a role in H. pylori infection. For example, other adhesins showed that a certain degree of IL-21 was improved via induced CD4+ T cells, and other adhesins could induce a high level of MMP families in AGS cells. Adhesins may play complex roles to affect immunocytes and gastric mucosa cells even throughout the process from adherence to invasion. This research only showed a point of view to explain the phenomenon based on adhesin HpaA and AGS migration. However, the HpaA binding to receptors and the binding to cause pathways activation are still unknown and need to be explored in future research.
5.1. Conclusions
Altogether, our results showed that adhesin HpaA increases MMP-2 and MMP-9 expressions of AGS cells by inducing the IL-21 secretion by CD4+ T cells. The findings indicated that adhesin HpaA plays a significant role through IL-21 in induced T cells during gastric mucosa injury or gastric cancer metastasis caused by H. pylori infection. Based on our research, HpaA may be a good therapeutic target, and aptamer HA6 a novel prevention strategy for H. pylori.
Acknowledgements
References
-
1.
Salimzadeh L, Bagheri N, Zamanzad B, Azadegan-Dehkordi F, Rahimian G, Hashemzadeh-Chaleshtori M, et al. Frequency of virulence factors in Helicobacter pylori-infected patients with gastritis. Microb Pathog. 2015;80:67-72. [PubMed ID: 25656240]. https://doi.org/10.1016/j.micpath.2015.01.008.
-
2.
Hatakeyama M. Malignant Helicobacter pylori-associated diseases: gastric cancer and malt lymphoma. Adv Exp Med Biol. 2019;1149:135-49. [PubMed ID: 31016622]. https://doi.org/10.1007/5584_2019_363.
-
3.
Falkeis-Veits C, Vieth M. Non-malignant Helicobacter pylori-associated diseases. Adv Exp Med Biol. 2019;1149:121-34. [PubMed ID: 31016630]. https://doi.org/10.1007/5584_2019_362.
-
4.
Huang JY, Goers Sweeney E, Guillemin K, Amieva MR. Multiple acid sensors control helicobacter pylori colonization of the stomach. PLoS Pathog. 2017;13(1). e1006118. [PubMed ID: 28103315]. [PubMed Central ID: PMC5245789]. https://doi.org/10.1371/journal.ppat.1006118.
-
5.
Tafreshi M, Guan J, Gorrell RJ, Chew N, Xin Y, Deswaerte V, et al. Helicobacter pylori type iv secretion system and its adhesin subunit, CagL, mediate potent inflammatory responses in primary human endothelial cells. Front Cell Infect Microbiol. 2018;8:22. [PubMed ID: 29468142]. [PubMed Central ID: PMC5808116]. https://doi.org/10.3389/fcimb.2018.00022.
-
6.
Bonsor DA, Zhao Q, Schmidinger B, Weiss E, Wang J, Deredge D, et al. The Helicobacter pylori adhesin protein HopQ exploits the dimer interface of human CEACAMs to facilitate translocation of the oncoprotein CagA. EMBO J. 2018;37(13). [PubMed ID: 29724755]. [PubMed Central ID: PMC6028028]. https://doi.org/10.15252/embj.201798664.
-
7.
Bonsor DA, Sundberg EJ. Roles of adhesion to epithelial cells in gastric colonization by Helicobacter pylori. Adv Exp Med Biol. 2019;1149:57-75. [PubMed ID: 31016628]. https://doi.org/10.1007/5584_2019_359.
-
8.
Ansari S, Yamaoka Y. Helicobacter pylori BabA in adaptation for gastric colonization. World J Gastroenterol. 2017;23(23):4158-69. [PubMed ID: 28694656]. [PubMed Central ID: PMC5483490]. https://doi.org/10.3748/wjg.v23.i23.4158.
-
9.
Lundstrom AM, Blom K, Sundaeus V, Bolin I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb Pathog. 2001;31(5):243-53. [PubMed ID: 11710844]. https://doi.org/10.1006/mpat.2001.0466.
-
10.
Zhang R, Wang C, Cheng W, Duan G, Shi Q, Chen S, et al. Delivery of Helicobacter pylori HpaA to gastrointestinal mucosal immune sites using Lactococcus lactis and its immune efficacy in mice. Biotechnol Lett. 2018;40(3):585-90. [PubMed ID: 29299716]. https://doi.org/10.1007/s10529-017-2502-3.
-
11.
Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5(9):688-98. [PubMed ID: 16138102]. https://doi.org/10.1038/nri1688.
-
12.
Caruso R, Fina D, Peluso I, Stolfi C, Fantini MC, Gioia V, et al. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells. Gastroenterology. 2007;132(1):166-75. [PubMed ID: 17241869]. https://doi.org/10.1053/j.gastro.2006.09.053.
-
13.
King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265-77. [PubMed ID: 15084263]. https://doi.org/10.1016/s0092-8674(04)00335-6.
-
14.
Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, et al. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J Immunol. 2007;178(9):5957-65. [PubMed ID: 17442980]. https://doi.org/10.4049/jimmunol.178.9.5957.
-
15.
Bagheri N, Azadegan-Dehkordi F, Shirzad M, Zamanzad B, Rahimian G, Taghikhani A, et al. Mucosal interleukin-21 mRNA expression level is high in patients with Helicobacter pylori and is associated with the severity of gastritis. Cent Eur J Immunol. 2015;40(1):61-7. [PubMed ID: 26155185]. [PubMed Central ID: PMC4472541]. https://doi.org/10.5114/ceji.2015.50835.
-
16.
Hua Y, Zhou F, Chen L, Li S, Tang X. [HpaA promotes gastric mucosa injury via stimulating IL-21 secretion of T cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35(8):744-9. [PubMed ID: 31638572].
-
17.
Spriestersbach A, Kubicek J, Schafer F, Block H, Maertens B. Purification of His-tagged proteins. Methods Enzymol. 2015;559:1-15. [PubMed ID: 26096499]. https://doi.org/10.1016/bs.mie.2014.11.003.
-
18.
Lenshof A, Jamal A, Dykes J, Urbansky A, Astrand-Grundstrom I, Laurell T, et al. Efficient purification of CD4+ lymphocytes from peripheral blood progenitor cell products using affinity bead acoustophoresis. Cytometry A. 2014;85(11):933-41. [PubMed ID: 25053536]. https://doi.org/10.1002/cyto.a.22507.
-
19.
Hua Y, Chen L, Li S, Zhou F, Tang X. [Screening and characterization of nucleic acid aptamers specifically binding to adhesin HpaA from Helicobacter pylori]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35(6):533-9. [PubMed ID: 31292058].
-
20.
Monteleone G, Pallone F, Macdonald TT. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth Factor Rev. 2009;20(2):185-91. [PubMed ID: 19261537]. https://doi.org/10.1016/j.cytogfr.2009.02.002.
-
21.
Tarnawski AS, Ahluwalia A. Molecular mechanisms of epithelial regeneration and neovascularization during healing of gastric and esophageal ulcers. Curr Med Chem. 2012;19(1):16-27. [PubMed ID: 22300072]. https://doi.org/10.2174/092986712803414088.
-
22.
Kim SJ, Park YS, Paik HD, Chang HI. Effect of anthocyanins on expression of matrix metalloproteinase-2 in naproxen-induced gastric ulcers. Br J Nutr. 2011;106(12):1792-801. [PubMed ID: 21733337]. https://doi.org/10.1017/S000711451100242X.
-
23.
Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S. Hydrogen peroxide-mediated downregulation of matrix metalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic Biol Med. 2006;41(6):911-25. [PubMed ID: 16934674]. https://doi.org/10.1016/j.freeradbiomed.2006.04.022.
-
24.
Chakraborty S, Stalin S, Das N, Choudhury ST, Ghosh S, Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33(10):2991-3001. [PubMed ID: 22257724]. https://doi.org/10.1016/j.biomaterials.2011.12.037.
-
25.
Xu CX, Zhong H, Shen SR. [Helicobacter pylori infection, the invasion and metastasis of the gastric carcinoma, and the expression of MMP-2 and TIMP-2]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29(6):643-6. [PubMed ID: 16114547].
-
26.
Bagheri N, Sadeghiani M, Rahimian G, Mahsa M, Shafigh M, Rafieian-Kopaei M, et al. Correlation between expression of MMP-9 and MMP-3 in Helicobacter pylori infected patients with different gastroduodenal diseases. Arab J Gastroenterol. 2018;19(4):148-54. [PubMed ID: 30509760]. https://doi.org/10.1016/j.ajg.2018.11.001.