Abstract
Background:
Candida albicanss has been introduced as one of the most common causes of nosocomial infections. Molecular typing methods are powerful tools in epidemiology to investigate the infection source of candidiasis, identify the transmission routes, and control the measures.Objectives:
This study aimed for genotyping C. albicans species isolated from oral cavities of the non-HIV patients who suffer from oropharyngeal candidiasis via combined ABC and repeat sequences (RPS) typing systems.Methods:
In this study, 31 DNA samples of clinical isolates of C. albicans were evaluated in terms of 25s ribosomal DNA region sequence or ABC typing, and ALT repeats numbers within RPS. DNA was amplified in two separate reactions, and the PCR products were electrophoresed to identify the genotypes of the isolates. Based on the band's pattern, phylogenetic analysis was conducted by UPGMA, and the discriminatory power of ABC and RPS typing was measured by Simpson’s index of diversity.Results:
Genotype A with (14 isolates, 45.2%) were the most frequent and followed by genotype B (10 isolates, 32.3%) and Genotype C (7 isolates, 22.6%), respectively. In addition, genotype 3 with 25 isolates (80.6%) were the most prevalent, followed by genotype 2/3 (4 isolates, 12.9%) and genotype 3/4 (2 isolates, 6.5%) respectively. No significant relationship was found between the obtained genotypes and drug-resistant isolates (P < 0.05).Conclusions:
This study showed that 25s rDNA and RPS typing is a quick, simple, and cost-effective method with average discriminatory power and good reproducibility for C. albicans genotyping. It can be used for the epidemiology of C. albicans infections.Keywords
1. Background
The incidence of Candida-induced infections has increased worldwide with high mortality rates in immunocompromised patients. These highly-prevalent yeasts, as a symbiotic microorganism and opportunistic pathogen, are responsible for a wide range of superficial, cutaneous, mucosal, and systemic infections (1). Recently, Candida has been introduced as the sixth most common cause of nosocomial infections (2). Candida species consists of a heterogeneous group of yeasts among which Candida albicans are known as the most prevalent species isolated from patients and healthy individuals (3-5). Despite the recent increase of non-albicans infections such as C. glabrata, C. tropicalis, the results of several studies indicate that over 80% of human infections caused by Candida species are associated with C. albicans. Studies have demonstrated that fungemia is associated with non-albicans Candida species (6-8). Moreover, mucocutaneous candidiasis is one of the most common fungal infections with C. albicans as its main pathogen. However, in recent decades non-albicans infections have increased (9, 10).
Currently, different typing methods are used to study population structure and species biodiversity, identify infection source and host-parasite relationship, determine and control the drug-resistant strains, and investigate the genetic link between the strains in epidemiologic studies. The most common typing methods are based on molecular advances, DNA fingerprinting, and genotyping. Each method should be evaluated in terms of ease of performance, reproducibility, discriminatory power, and interpretation (1). With high discriminatory power, genotyping methods are powerful tools in epidemiology to investigate the infection source of candidiasis, identify the transmission routes, and control measures (11, 12).
Ribosomal sequencing, and examining PCR products of 25s rDNA region have been frequently used in genotyping of C. albicans (13). In this method, based on the electrophoresis banding pattern, C. albicans is divided into several genotypes, including A, B, C, D, and E, among which genotype A has been reported as the dominant genotype in different regions (11, 13, 14). A study has reported that the combined analysis of 25s rDNA and repeated sequences (RPS) could increase the discriminatory power of C. albicans genotyping (10). In this method, a combination of microsatellite markers is used in strain typing within different chromosomes (15). Studies indicate that C. albicans consists of RPS in all its chromosomes except chromosome 3 (16-18). Each RPS region has a tandem short repeating unit, such as 172 bp known as ALT (16, 19, 20). The number of alternative lengthening of telomerase (ALT) repetitions in RPS differs in each chromosome, leading to diversity in RPSs fragment size (21). Consequently, subunits can be identified, which is an intriguing target for C. albicans genotyping (20). Based on the size difference of RPS and the number of copies of ALT, C. albicans is divided into four groups: Aa, Ab, Ac, and Ad. Moreover, it has been confirmed that genotype D is associated with C. dubliniensis (22, 23).
2. Objectives
This study aims for genotyping and identifying the population structure of C. albicans species isolated from oral cavities of the patients suffering oropharyngeal candidiasis lesions based on ABC and RPS typing systems.
3. Methods
In this study, 31 DNA samples of clinical isolates of C. albicans were collected from patients with oropharyngeal candidiasis who went to Cancer Institute of Tehran, Tehran, Iran. Then, they were evaluated in terms of ABC and RPS genotyping systems (24). The pair primers of CA-INT-L and CA-INT-R were used.to determine the genotyping of C. albicans based on 25s rDNA region sequence. Besides, pair primers of ASDcF and pCSCR were utilized to determine ALT repetitions within RPS (17, 23). Table 1 shows the nucleotide sequence of primers and molecular size of the PCR product (11). DNA was amplified in a reaction mixture (25 µL) containing 12.5 µL of master mix (SinaClon Bioscience Co., Karaj, Iran), 1 µL (4 ng) of DNA template, 1.5 µL of a forward primer (5 mM), 1.5 µL of a reverse primer (5 mM), and 8.5 µL of distilled water. The PCR cycle parameters were as follows: initial denaturation at 97ºC for 7 min, followed by 30 cycles of 94ºC for 30 sec, 60ºC for 30 sec and 72ºC for 40 sec and final extension 72ºC for 5 min. All reaction mixtures were amplified using a thermal cycler (SimpliAmp; Applied biosystem; Cat No: A24811). Electrophoresis of the PCR product was performed using 1.5% agarose gel in TBE buffer. Band pattern of electrophoresis agarose gel was converted to a zero-one matrix.
Characteristics of Primers for the 25s rDNA, ALT Region, and Expected Bands
| Primers | Sequence | Target | Expected amplicon size (bp) |
|---|---|---|---|
| CA-INT-L | 5’-ATAAGGGAAGTCGGCAAAATAGATCCG-3’ | 25s rDNA | A-450; B-840; C-450, 840; D-1040; E-1080 |
| CA-INT-R | 5’-CTTGGCTGTGGTTTCGCTAGATAGTAGAT-3’ | ||
| ASDcF | 5’-TGATGAACCACATGTGCTACAAAG-3’ | RPS | 1- 526; 2- 698; 3- 870; 4- 1042; 5- 1214; 6- 1396 |
| pCSCR | 5’-CGCCTCTATTGGTCGAGCAGTAGTC-3’ |
3.1. Phylogenetic Analysis
Electrophoresis band pattern data was converted to zero-one matrix. Then, phylogenetic analysis was conducted by the unweighted pair group method with arithmetic averages (UPGMA) via an online tool (www.genoms.urv.cat/upgma) applied to zero-one matrix data. The discriminatory power of ABC and RPS typing was measured by Simpson’s index of diversity (25), which demonstrates the capacity of a typing method to differentiate between species. To calculate the discriminatory power, an online tool (http://insilico.ehu.eus/) was used.
4. Results
The PCR product using 25s rDNA primer was obtained of 450 bp for genotype A, 840 bp for genotype B, and the two bands of 840 bp and 450 bp for genotype C. Genotype A with 14 isolates (45.2%) were the most frequent followed by genotype B (10 isolates, 32.3%) and genotype C (7 isolates, 22.6%) respectively. Also, the banding pattern of RPS typing showed that genotype 3 was the most prevalent (25 isolates, 80.6%), followed by genotype 2/3 (4 isolates, 12.9%) and genotype 3/4 (2 isolates, 6.5%) respectively. The frequency of genotypes in the combination of the ABC and RPS typing is shown in Table 2.
The Frequency of Genotypes Candida albicans Clinical Isolates in the Combination of the ABC and RPS Typing System, Based on the Result Genotype A3 is the Most Prevalent Genotype
| ABC Typing | RPS Typing | ||
|---|---|---|---|
| Genotype 3 (%) | Genotype 2/3 (%) | Genotype 3/4 (%) | |
| Type A | 13 (41.9) | 1 (3.2) | 0 (0.0) |
| Type B | 6 (19.35) | 2 (6.5) | 2 (6.5) |
| Type C | 6 (19.35) | 1 (3.2) | 0 (0.0) |
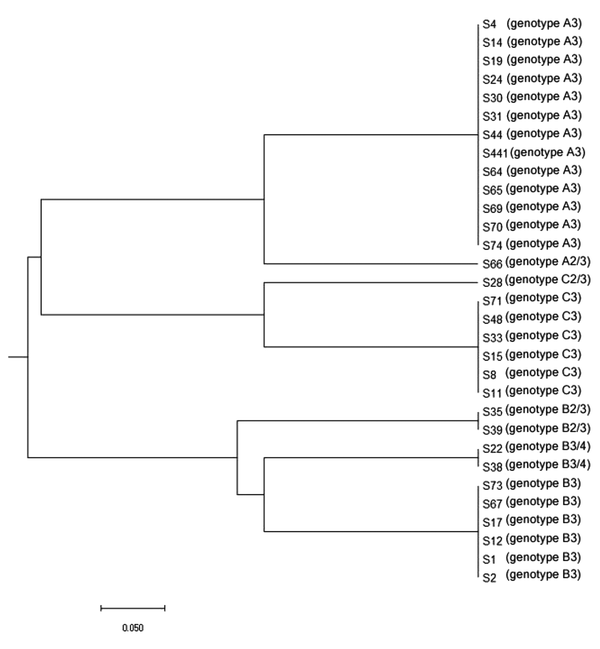
UPGMA dendrogram was constructed based on the combination of ABC and RPS typing system data using online tool (www.genoms.urv.cat/upgma), as shown in Figure 1. The discriminatory power of ABC and RPS typing system using Simpson’s index of diversity was conducted through an online tool (http://insilico.ehu.eus/) (25). The results showed that the 31 clinical isolates of C. albicans were classified into 7 distinct genotypes, indicating a discriminatory power index of 0.7634.
Dendrogram shows the genetic relationships between 31 isolates of Candida albicans. The dendrogram was constructed by zero-one matrix data from the combinations of ABC and RPS typing system and using the UPGMA method.
5. Discussion
In this study, the ABC and RPS typing of C. albicans clinical isolates were carried out based on electrophoresis pattern of polymorphism. Since the ABC typing method cannot fully distinguish between strains, the fragment analysis was used via the combined microsatellite markers of ABC and RPS typing system for more successful typing of C. albicans. RPS typing results of 25s rDNA region indicated that genotype A was the dominant genotype with 14 isolates (45.2%) followed by genotypes B and C with 10 (32.3%) and 7 (22.6%) isolates, respectively. The study results were in agreement with various reports from all over the world; genotype A has been reported as the dominant genotype (10, 11, 13, 23, 24, 26). Dalvand et al. (2018) reported that out of 27 C. albicans strains isolated from animal cases, 11, 6, 5, and 5 cases were recognized as genotypes A (40.8%), E (22.2%), B (18.5%), and C (18.5%), respectively. Iwata et al. (2006) reported that out of 179 strains of C. albicans, 92 isolates (51.4%) of genotype A, 49 isolates (27.3%) of genotype C and 38 isolates (21.2%) of genotype B were obtained (10).
Tamai et al. (2014) investigated C. albicans strains isolated from AIDS patients via ABC typing, and reported genotype A as the predominant genotype with 66% of frequency, followed by genotype B and genotype C with 24% and 10% respectively (26). The ratio of genotypes A, B, and C is not the same in different studies due to the clinical specimens, geographical areas, and different population of patients. In addition, the band pattern obtained from RPS (ALT) typing indicated that genotype 3 had the highest frequency with 25 isolates (80.6%) followed by genotypes 2/3 and 3/4 with 4 (12.9%) and 2 (6.5%) isolates, respectively. These results are also in agreement with the previous findings (10, 11). Some studies have reported that in RPS typing of C. albicans, genotype 3, and 3/4 are the predominant clinical isolates (10, 11).
It has been reported that RPS diagnostic and typing method based on the number of sequence repetitions of RPS (ALT) can be used not only for C. albicans genotyping but also for the identification and differentiation of C. albicans from C. stellatoidea and C. dubliniensis (10, 18, 22). C. stellatoidea and C. dubliniensis are known as pathogenic species that are closely related to C. albicans (18). however C. stellatoidea and C. dubliniensis were not isolated in this study. The RPS typing system has been introduced as a simple, quick, and efficient method for C. albicans genotyping (10, 11, 20). Unlike some methods, such as RAPD-PCR, this method has a proper reproducibility rarely affected by laboratory conditions (27). The study of Iwata et al. in 2006 showed that the RPS typing system was more appropriate than PFGE for the differentiation of C. albicans strains (10). However, discriminatory power (D.P) of samples indicated that 31 clinical isolates of C. albicans via RPS typing system were divided into 7 distinct genotypes with a D.P of 0.7634, while MLST genotyping of these samples (28) showed that 31 clinical isolates of C. albicans were classified into 29 different genotypes with D.P of 0.9957, indicating a much higher discriminatory power compared to RPS typing system.
MLST is a powerful method in the genotyping of Candida species with high discriminatory power and clear results (29-32). MLST is the only genotyping method, which allows global surveillance and comparison of C. albicans, C. tropicalis, C. glabrata, C. dubliniensis, and C. krusei genotypes in a central web-based database. Thus, the possibility of epidemiological studies at the international level is provided. However, the high cost of nucleotide sequencing precludes public use of this method. In general, despite their deficiencies used in small-scale epidemiological studies, qualitative methods such as RAPD, PFGE, and RPS are affordable and time-saving, particularly for invasive nosocomial candidiasis with low sample size. Therefore, the typing method should be selected based on the purpose of study and facilities (33).
5.1. Conclusions
This study showed that 25s rDNA and RPS typing is a quick, simple, and cost-effective method with average discriminatory power for C. albicans genotyping and managing C. albicans infections.
References
-
1.
Trtkova J, Raclavsky V. Molecular-genetic approaches to identification and typing of pathogenic Candida yeasts. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150(1):51-61. [PubMed ID: 16936901].
-
2.
Pérez-García LA, Macías-Pérez JR, León-Buitimea Á, Alvarado-Sánchez B, Ramírez-Quijas MD, Navarro-Arias MJ, et al. Candida and Candidiasis. In: Mora-Montes HM, Lopes-Bezerra LM, editors. Current Progress in Medical Mycology. Cham: Springer International Publishing; 2017. p. 91-118. https://doi.org/10.1007/978-3-319-64113-3_3.
-
3.
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133-63. [PubMed ID: 17223626]. [PubMed Central ID: PMC1797637]. https://doi.org/10.1128/CMR.00029-06.
-
4.
Kord Z, Fata A, Zarrinfar H. Molecular Identification of Candida species isolated from patients with vulvovaginitis for the first time in Mashhad. Iran J Obstet Gynecol Infertil. 2017;20(4):50-7. https://doi.org/10.22038/ijogi.2017.8982.
-
5.
Zarrinfar H, Kaboli S, Dolatabadi S, Mohammadi R. Rapid detection of Candida species in bronchoalveolar lavage fluid from patients with pulmonary symptoms. Braz J Microbiol. 2016;47(1):172-6. [PubMed ID: 26887241]. https://doi.org/10.1016/j.bjm.2015.02.001.
-
6.
Colombo AL, Guimaraes T, Silva LR, de Almeida Monfardini LP, Cunha AK, Rady P, et al. Prospective observational study of candidemia in Sao Paulo, Brazil: incidence rate, epidemiology, and predictors of mortality. Infect Control Hosp Epidemiol. 2007;28(5):570-6. [PubMed ID: 17464917]. https://doi.org/10.1086/513615.
-
7.
Chakrabarti A, Chatterjee SS, Rao KL, Zameer MM, Shivaprakash MR, Singhi S, et al. Recent experience with fungaemia: change in species distribution and azole resistance. Scand J Infect Dis. 2009;41(4):275-84. [PubMed ID: 19229762]. https://doi.org/10.1080/00365540902777105.
-
8.
Esmailzadeh A, Zarrinfar H, Fata A, Sen T. High prevalence of candiduria due to non-albicans Candida species among diabetic patients: A matter of concern? J Clin Lab Anal. 2018;32(4):e22343. [PubMed ID: 29076587]. https://doi.org/10.1002/jcla.22343.
-
9.
Rogers PD, Barker KS. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob Agents Chemother. 2003;47(4):1220-7. [PubMed ID: 12654650]. [PubMed Central ID: PMC152536]. https://doi.org/10.1128/aac.47.4.1220-1227.2003.
-
10.
Iwata T, Hattori H, Chibana H, Mikami Y, Tomita Y, Kikuchi A, et al. Genotyping of Candida albicans on the basis of polymorphisms of ALT repeats in the repetitive sequence (RPS). J Dermatol Sci. 2006;41(1):43-54. [PubMed ID: 16364601]. https://doi.org/10.1016/j.jdermsci.2005.08.010.
-
11.
Hattori H, Iwata T, Nakagawa Y, Kawamoto F, Tomita Y, Kikuchi A, et al. Genotype analysis of Candida albicans isolates obtained from different body locations of patients with superficial candidiasis using PCRs targeting 25S rDNA and ALT repeat sequences of the RPS. J Dermatol Sci. 2006;42(1):31-46. [PubMed ID: 16414246]. https://doi.org/10.1016/j.jdermsci.2005.12.003.
-
12.
Arastehfar A, Daneshnia F, Hafez A, Khodavaisy S, Najafzadeh M, Charsizadeh A, et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med Mycol. 2019. https://doi.org/10.1093/mmy/myz124.
-
13.
Millar BC, Moore JE, Xu J, Walker MJ, Hedderwick S, McMullan R. Genotypic subgrouping of clinical isolates of Candida albicans and Candida dubliniensis by 25S intron analysis. Lett Appl Microbiol. 2002;35(2):102-6. https://doi.org/10.1046/j.1472-765X.2002.01135.x.
-
14.
Lian C, Zhao J, Zhang Z, Liu W. Genotype of Candida species associated with different conditions of vulvovaginal candidosis. Mycoses. 2004;47(11-12):495-502. [PubMed ID: 15601456]. https://doi.org/10.1111/j.1439-0507.2004.01049.x.
-
15.
Adachi H, Shimizu K, Hattori H, Tanaka R, Chibana H, Takagi Y, et al. Genotyping of Candida albicans by fragment analysis of microsatellites combined with 25S rDNA and RPS-based strategies. Nihon Ishinkin Gakkai Zasshi. 2009;50(3):167-74. [PubMed ID: 19654450].
-
16.
Iwaguchi S, Homma M, Chibana H, Tanaka K. Isolation and characterization of a repeated sequence (RPS1) of Candida albicans. J Gen Microbiol. 1992;138(9):1893-900. [PubMed ID: 1402790]. https://doi.org/10.1099/00221287-138-9-1893.
-
17.
Chibana H, Iwaguchi S, Homma M, Chindamporn A, Nakagawa Y, Tanaka K. Diversity of tandemly repetitive sequences due to short periodic repetitions in the chromosomes of Candida albicans. J Bacteriol. 1994;176(13):3851-8. [PubMed ID: 8021166]. [PubMed Central ID: PMC205581]. https://doi.org/10.1128/jb.176.13.3851-3858.1994.
-
18.
Hattori H, Tanaka R, Chibana H, Kawamoto F, Adachi H, Shimizu K, et al. Improvement of the repetitive sequence-based identification and genotyping of Candida albicans using ALT-specific primers. Jpn J Infect Dis. 2009;62(3):215-9. [PubMed ID: 19468185].
-
19.
Chindamporn A, Nakagawa Y, Homma M, Chibana H, Doi M, Tanaka K. Analysis of the chromosomal localization of the repetitive sequences (RPSs) in Candida albicans. Microbiology. 1995;141 ( Pt 2):469-76. [PubMed ID: 7704277]. https://doi.org/10.1099/13500872-141-2-469.
-
20.
Mijiti J, Pu XM, Erfan A, Yaguchi T, Chibana H, Tanaka R. Genotyping of fluconazole-resistant Candida albicans isolated from Uighurian people in Xinjing (China) using ALTS/RFLP and micro-TGGE method. Nihon Ishinkin Gakkai Zasshi. 2010;51(3):165-8. [PubMed ID: 20716855].
-
21.
Ranjbar R, Karami A, Farshad S, Giammanco GM, Mammina C. Typing methods used in the molecular epidemiology of microbial pathogens: a how-to guide. New Microbiol. 2014;37(1):1-15. [PubMed ID: 24531166].
-
22.
Kanbe T, Kurimoto K, Hattori H, Iwata T, Kikuchi A. Rapid identification of Candida albicans and its related species Candida stellatoidea and Candida dubliniensis by a single PCR amplification using primers specific for the repetitive sequence (RPS) of Candida albicans. J Dermatol Sci. 2005;40(1):43-50. [PubMed ID: 16054340]. https://doi.org/10.1016/j.jdermsci.2005.06.007.
-
23.
McCullough MJ, Clemons KV, Stevens DA. Molecular and phenotypic characterization of genotypic Candida albicans subgroups and comparison with Candida dubliniensis and Candida stellatoidea. J Clin Microbiol. 1999;37(2):417-21. [PubMed ID: 9889231]. [PubMed Central ID: PMC84325].
-
24.
Karahan ZC, Guriz H, Agirbasli H, Balaban N, Gocmen JS, Aysev D, et al. Genotype distribution of Candida albicans isolates by 25S intron analysis with regard to invasiveness. Mycoses. 2004;47(11-12):465-9. [PubMed ID: 15601450]. https://doi.org/10.1111/j.1439-0507.2004.01022.x.
-
25.
Hunter PR. Reproducibility and indices of discriminatory power of microbial typing methods. J Clin Microbiol. 1990;28(9):1903-5. [PubMed ID: 2229371]. [PubMed Central ID: PMC268075].
-
26.
Tamai IA, Salehi TZ, Sharifzadeh A, Shokri H, Khosravi AR. Repetitive sequences based on genotyping of Candida albicans isolates obtained from Iranian patients with human immunodeficiency virus. Iran J Basic Med Sci. 2014;17(11):831-5. [PubMed ID: 25691923]. [PubMed Central ID: PMC4328090].
-
27.
Dalvand A, Katiraee F, Jafari Joozani R, Shokri H. Genotyping of Candida albicans isolated from animals using 25S ribosomal DNA and ALT repeats polymorphism in repetitive sequence. Curr Med Mycol. 2018;4(4):12-9. [PubMed ID: 30815612]. [PubMed Central ID: PMC6386504]. https://doi.org/10.18502/cmm.4.4.381.
-
28.
Muller FM, Werner KE, Kasai M, Francesconi A, Chanock SJ, Walsh TJ. Rapid extraction of genomic DNA from medically important yeasts and filamentous fungi by high-speed cell disruption. J Clin Microbiol. 1998;36(6):1625-9. [PubMed ID: 9620390]. [PubMed Central ID: PMC104890].
-
29.
Robles JC, Koreen L, Park S, Perlin DS. Multilocus sequence typing is a reliable alternative method to DNA fingerprinting for discriminating among strains of Candida albicans. J Clin Microbiol. 2004;42(6):2480-8. [PubMed ID: 15184424]. [PubMed Central ID: PMC427821]. https://doi.org/10.1128/JCM.42.6.2480-2488.2004.
-
30.
Dodgson AR, Pujol C, Denning DW, Soll DR, Fox AJ. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J Clin Microbiol. 2003;41(12):5709-17. [PubMed ID: 14662965]. [PubMed Central ID: PMC309006]. https://doi.org/10.1128/jcm.41.12.5709-5717.2003.
-
31.
Katiraee F, Khalaj V, Khosravi AR, Hajiabdolbaghi M. Sequences type analysis of Candida albicans isolates from Iranian human immunodeficiency virus infected patients with oral candidiasis. Acta Med Iran. 2014;52(3):187-91. [PubMed ID: 24901719].
-
32.
Afsarian MH, Badali H, Boekhout T, Shokohi T, Katiraee F. Multilocus sequence typing of Candida albicans isolates from a burn intensive care unit in Iran. J Med Microbiol. 2015;64(Pt 3):248-53. [PubMed ID: 25596113]. https://doi.org/10.1099/jmm.0.000015.
-
33.
De Valk HA, Klaassen CHW, Meis JFGM. Molecular typing of Aspergillus species. Mycoses. 2008;51(6):463-76. https://doi.org/10.1111/j.1439-0507.2008.01538.x.
