1. Background
Dacryocystitis is an infection of the nasolacrimal sac caused by nasolacrimal duct obstruction. Obstruction of the nasolacrimal duct leads to mucous secretion in the lacrimal sac. In this situation, microorganisms, such as normal flora or pathogens, can be colonized within the lacrimal sac (1). Clinical symptoms of dacryocystitis include pain, redness, and swelling over the inner side of the lower eyelid (2). The microbial agents of this disease have not been described in different geographical areas. Staphylococcus spp., Streptococcus pneumoniae, and Pseudomonas aeruginosa are the most common causes of dacryocystitis (1). Studies reported that Staphylococcus aureus, coagulase-negative staphylococci (CoNS), Streptococcus spp., and Gram-negative bacilli were the most frequently isolated bacteria (3). Moreover, antibacterial susceptibility tests showed that Gram-positive and Gram-negative bacteria were most sensitive to rifampicin, gentamicin, and amikacin (3, 4).
2. Objectives
This study was performed to determine bacterial pathogen agents of dacryocystitis and its antimicrobial susceptibility patterns in patients. To this end, we examined the microbiology and antibiotic susceptibility of ocular samples to provide the best treatment for dacryocystitis.
3. Methods
3.1. Study Design
This cross-sectional study was performed from June 2017 to June 2018 on patients with dacryocystitis admitted to the ophthalmology clinic of the Matini Hospital, Kashan, Iran. An ophthalmologist examined all the patients to see whether they had clinical signs of duct obstruction. The patients completed a demographic questionnaire on age, sex, place of residence, and occupation. Furthermore, information concerning the type of disease, the form of discharge, clinical signs, and background illness was obtained from the patients.
3.2. Bacterial Isolation
Swabs taken from the patients were collected in the sterile tryptic soy broth medium (Merck, Germany). The specimens were transported to the microbiology laboratory of the Kashan University of Medical Sciences immediately. One of the swabs was used for Gram staining, and the other was inoculated on a blood agar with 5% sheep blood, MacConkey agar, and chocolate agar (Merck, Germany). The media were inoculated under different conditions: (1) at 37°C for 24 h in aerobic conditions; (2) at 37°C in the presence of 10% CO2 in a candle jar for 48 h; and (3) at 37°C in an anaerobic jar loaded with Gaspak for 48 h.
The isolates were identified based on microscopic morphology, staining characteristics, and cultural and biochemical properties using oxidase, catalase, coagulase, DNase, optochin, CAMP, IMViC, and susceptibility to bile tests, growth at mannitol salt agar and 6.5% NaCl (Merck, Germany). The antibiotic susceptibility test was performed using the Kirby-Bauer method according to the Clinical and Laboratory Standards Institute (CLSI) protocol (1). Finally, the plate was incubated at 37ºC for 24 h. The zone of inhibition around the disk was measured and compared to CLSI 2018 charts (5). The antibiotics used for antimicrobial susceptibility are shown in Table 1 (Mast, UK).
| Gram-Positive Bacteria | Gram-Negative Bacteria | Non-fermented Gram-Negative Bacteria |
|---|---|---|
| PG: penicillin (10 µg) | AK: amikacin (30 µg) | AK: amikacin (30 µg) |
| Fox: cefoxitin (30 µg) | GM: gentamicin (10 μg) | GM: gentamicin (10 μg) |
| GM: gentamicin (10 μg) | TS: cotrimoxazole (25 µg) | CAZ: ceftazidime (30 µg) |
| E: erythromycin (15 µg) | CIP: ciprofloxacin (5 µg) | MEM: meropenem (10 μg) |
| T: tetracycline (30 μg) | CTX: cefotaxime (30 µg) | TS: cotrimoxazole (25 µg) |
| TS: cotrimoxazole (25 µg) | PTZ: piperacilin-tazobactam (110 µg) | ATM: aztreonam (30 µg) |
| RP: rifampicin (5 µg) | AUG: augmentin (30 µg) | CIP: ciprofloxacin (5 µg) |
| CIP: ciprofloxacin (5 µg) | AP: ampicillin (10 µg) | CTX: cefotaxime (30 µg) |
| LZD: linezolid (30 μg) | CAZ: ceftazidime (30 µg) |
3.3. Detection of Methicillin-Resistant Staphylococcus
In the current study, methicillin-resistant Staphylococcus (MRS) was detected using a cefoxitin disk. In interpreting the results, each growth inhibition zoon < 21 mm (for S. aureus) and < 24 mm (for CoNS) was considered a methicillin-resistant strain (6).
3.4. Detection of Extended-Spectrum Beta-Lactamase
The combination of ceftazidime disks alone and ceftazidime (30 μg)/clavulanic acid (10 μg) was placed at a space of 2 cm on Mueller-Hinton agar (Merck, Germany) was used for Enterobacteriaceae and non-fermented Gram-negative bacilli. Extended-spectrum beta-lactamase (ESBL) production was distinguished with an increased width about the disk of ceftazidime/clavulanic acid by 5 mm or more (7).
3.5. Molecular Identification Methicillin-Resistant Staphylococcus
3.5.1. DNA Extraction
The boiling method was used to extract DNA. For this purpose, bacteria isolates were cultured on the blood agar (Merck, Germany). Then, two loops filled with pure colonies were added to the sterile microtube containing 500 μL deionized DNase and RNase-free water and placed in boiling water for 15 min. Afterward, the samples were placed on ice for 2 - 3 min and centrifuged at 10,000 g for 7 min. The supernatant containing DNA was stored at -20°C. An amount of 4µL of this supernatant was applied as the template for PCR reaction.
3.5.2. PCR Reaction
Methicillin-resistant Staphylococcus isolates were detected by recognition of the mecA gene using the PCR technique. The characteristics of the used primers are shown in Table 2. The amplification condition for the mecA gene was an initial denaturation step at 94°C for 4 min, 35 cycles of denaturation at 94°C for 45 s, annealing at 50°C for 45 s, extension at 72°C for 1 min, and a final extension at 72°C for 2 min. Finally, the PCR products were run in 1% agarose gel. Also, S. aureus ATCC 33591 and S. epidermidis ATCC 12228 were used as the positive and negative controls, respectively.
| Primer | Primer Sequence | Amplicons Size (bp) | Ref. |
|---|---|---|---|
| mecA F | 5'- ACTGCTATCCACCCTCAAAC-3' | 160 | (8) |
| mecA R | 5'-CTGGTGAAGTTGTAATCTGG-3' |
3.6. Statistical Analysis
The frequency percentage of all the data was calculated. Chi-square distribution was used to test the qualitative distribution. A P-value < 0.05 was considered significant.
4. Results
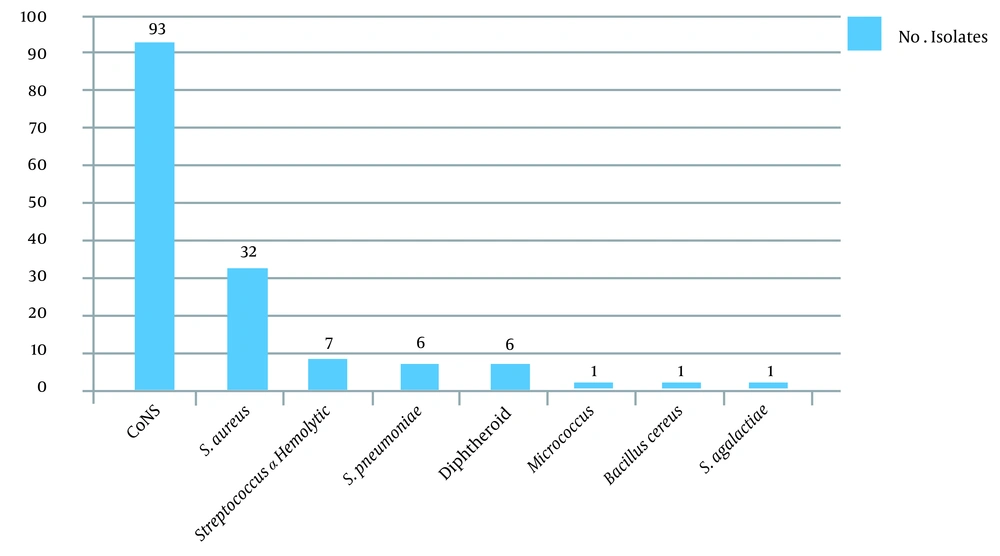
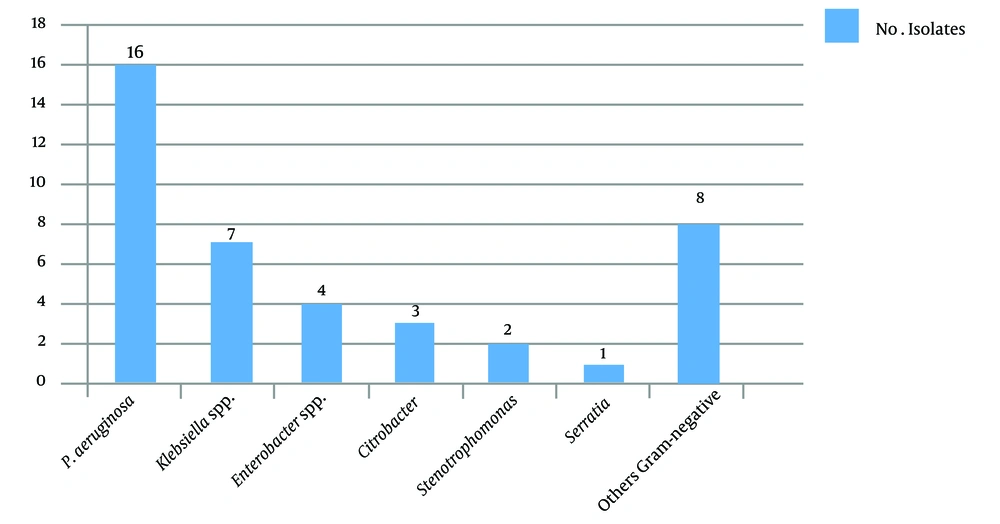
A total of 172 eye specimens were analyzed. Of the patients, 88.4% lived in urban areas, and 11.6% lived in rural areas. Moreover, 56.2 and 43.8% of the patients were male and female, respectively. Also, 82.9% of the patients were under 30 years of age, and 17.1% of them were between 30 - 61 years of age, with the minimum age being 6 days and the maximum age being 96 years. According to the symptoms, 30.3% of the patients had chronic dacryocystitis, and 69.7% of them had acute dacryocystitis. Further, 41.8% of the patients had purulent discharge, and 58.2% of them had mucous discharge. Swelling and redness were observed in 5.4% of the patients (100%). In this study, culture was positive in 96.9% of the specimens, and more than one bacterium strain were isolated from some of the specimens. Frequency of Gram-positive and Gram-negative bacterial agents isolated from dacryocystitis patients show in Figures 1 and 2.
The antibiotic susceptibility results are shown in Tables 3 - 5. Ceftazidime resistance was observed in ten isolates of Enterobacteriaceae and nine isolates of non-fermented Gram-negative bacilli. Using the combination disk test method, we detected ESBL activity in seven isolates (70%) of Enterobacteriaceae. The frequency of ESBL in non-fermented Gram-negative bacteria was two isolates (22.2%). Extended-spectrum beta-lactamase-producing bacteria were Klebsiella (n = 2), Yersinia (n = 2), Escherichia coli (n = 1), Citrobacter (n = 1), Enterobacteriaceae (n = 1), P. aeruginosa (n = 1), and Stenotrophomonas (n = 1). Of the 32 S. aureus isolated in this study, ten were confirmed as methicillin-resistant S. aureus (MRSA). Moreover, 66 of the 93 CoNS isolated were verified as methicillin-resistant CoNS (MR-CoNS) using the phenotyping method. Methicillin-resistant Staphylococcus isolates were recognized via identifying the mecA gene using the PCR method. The results showed that 40% (n = 4) of the MRSA isolates and 89.4% (n = 59) of the MR-CoNS isolates carried the mecA gene, respectively.
| Organism/Isolate | PG | FOX | GM | E | T | TS | RP | CIP | LZD |
|---|---|---|---|---|---|---|---|---|---|
| CoNS/93 | 13 (14) | 57 (61.3) | 84 (90.3) | 32 (34.4) | 60 (64.5) | 74 (79.6) | 88 (94.6) | 76 (81.7) | 91 (97.8) |
| Staphylococcus aureus/32 | 3 (9.4) | 22 (68.7) | 32 (100) | 15 (46.8) | 26 (81.25) | 26 (81.25) | 28 (87.5) | 30 (93.75) | 32 (100) |
| S. α hemolytic/7 | - | - | - | 3 (42.8) | 4 (57.1) | - | 7 (100) | - | 5 (71.4) |
| S. β hemolytic/1 | - | - | - | 1 (100) | - | - | 1 (100) | - | 1 (100) |
a Values are expressed as No. (%).
| Organisms/Isolate | AK | GM | CAZ | MEM | TS) | ATM | CIP) | CTX |
|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa/ 16 | 14 (87.5) | 13 (81.2) | 8 (50) | 12 (75) | - | 2 (12.5) | 12 (75) | - |
| Acinetobacter baumannii/ 7 | 5 (71.4) | 4 (57.1) | 1 (14.2) | 5 (71.4) | - | - | - | 4 (57.1) |
| Burkholderia/ 1 | - | - | 1 (100) | 1 (100) | 1 (100) | - | - | - |
a Values are expressed as No. (%).
| Organisms/Isolate | AK | GM | TS | CIP | CTX | PTZ | AUG | AP | CAZ |
|---|---|---|---|---|---|---|---|---|---|
| Enterobacterspp./3 | 2 (66.6) | 2 (66.6) | 1 (33.3) | 2 (66.6) | 2 (66.6) | 2 (66.6) | 2 (66.6) | 2 (66.6) | - |
| Klebsiellaspp./4 | 4 (100) | 4 (100) | 3 (75) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 2 (50) | 1 (25) |
| Escherichia coli/2 | 2 (100) | 2 (100) | 1 (50) | 2 (100) | 2 (100) | 1 (50) | 1 (50) | 1 (50) | 1 (25) |
| Yersinia enterocolitica/4 | 3 (75) | 4 (100) | 4 (100) | 4 (100) | 3 (75) | 3 (75) | 1 (25) | - | 2 (50) |
| Citrobacter/2 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | - | - | 1 (25) |
a Values are expressed as No. (%).
5. Discussion
Dacryocystitis is a common lacrimal duct infection. The lacrimal system directs tears from the lacrimal glands to the nasal cavity (9). Obstruction of the lacrimal duct causes tear retention and, consequently, bacterial accumulation in this area (10). In this study, 96.9% of the specimens had positive culture, and 12 species of bacteria were isolated. Our results demonstrated that CoNS and S. aureus were the most frequent Gram-positive bacteria, and P. aeruginosa was the most Gram-negative isolated organism in the patients. The results of several other studies confirm this finding (11, 12).
Women are more commonly affected by lacrimal duct obstruction because of the lower nasolacrimal duct and hormonal factors (13). In our study, the number of female patients was higher than male ones in the age groups 1 - 30 years and > 60 years. The reason may be prolonged presence in the kitchen, hot air, the use of different types of cosmetics, and a weakened immune system in old age. The present study results also showed that the disease was predominantly acute in the age groups < 1 year and 1 - 30 years and chronic in the age groups between 31 - 60 years and > 60 years (Table 6). Previous studies also showed that patients < 30 years had significantly acute dacryocystitis (4, 14). Identification of bacterial agents that cause dacryocystitis and their antibiotic sensitivity is essential in treating and preventing this complication. According to studies performed on the microbial profile of dacryocystitis, there has been considerable variation in microbial agents and drug management (11). However, during the past 50 years, the prevalence of microbial agents of dacryocystitis has changed. In the 1930s, S. pneumoniae was the most common isolated agent of this infection. However, today, it is much less frequent than S. epidermidis (1).
| Age (y) | Form of Disease | Gender | Common Organism | |||||
|---|---|---|---|---|---|---|---|---|
| Acute | Chronic | Acute | Chronic | Acute | Chronic | |||
| M | F | M | F | |||||
| < 1 | 103 (86) | 17 (14) | 52 (50.4) | 51 (49.5) | 10 (58.8) | 7 (41.2) | CoNS | CoNS |
| 1 - 30 | 20 (69) | 9 (31) | 6 (30) | 14 (70) | 4 (44.4) | 5 (55.5) | CoNS | CoNS, S. aureus |
| 31 - 60 | 2 (13.3) | 13 (86.6) | 2 (100) | 0 | 7 (53.8) | 6( 46.2) | CoNS | CoNS |
| > 60 | 5 (31.25) | 11 (68.75) | 1 (20) | 4 (80) | 3 (27.2) | 8 (72.7) | CoNS | S. aureus |
a Values are expressed as No. (%) unless otherwise indicated.
Nowadays, MRSA is frequently detected in ocular infections worldwide, and it has been determined that methicillin-resistant S. epidermidis can cause ophthalmic infections and blindness (15). In our study, the predominant organism in the acute and chronic forms of the disease was CoNS. In the age groups 1 - 30 years and > 60 years, in addition to CoNS, MRSA was also the cause of the disease (Table 6). In other studies, Gram-positive organisms were documented in 78.58% of organisms (16). Chaudhary reported that S. epidermidis (89.62%) was the most common isolate from lacrimal sac infection, followed by S. aureus (17).
In the current study, different antibiotics were used to study the microbial susceptibility of bacterial strains. In the group of Gram-positive bacteria, the most common isolated organism, CoNS, had sensitivity to linezolid (97.8%), rifampicin (94.6%), gentamicin (90.3%), and ciprofloxacin (81.7%). Also, S. aureus showed the highest susceptibility to linezolid (100%), gentamicin (100%), and ciprofloxacin (93.75%). Among all the analyzed P. aeruginosa isolates, the highest resistance was observed to aztreonam. The results of Negm et al.'s study confirm our results, showing that Gram-negative isolates were more sensitive to the third generation of cephalosporins, such as cefotaxime (80%) and ceftazidime (60%) (2). In the Enterobacteriaceae group, the highest (100%) sensitivity was to amikacin, gentamicin, ciprofloxacin, cefotaxime, piperacillin-tazobactam, and augmentin in Klebsiella. Thus, these drugs can be used to treat dacryocystitis caused by Klebsiella strains. However, sensitivity to cefotaxime, ciprofloxacin, amikacin, and gentamicin was the highest (100%) in the E. coli group (Tables 3 - 5). Sun et al. showed that among aminoglycosides, amikacin had the highest effectiveness toward Gram-negative isolates (18), which agrees with our results. Also, the highest resistance in this group was to ampicillin and ceftazidime in Klebsiella and augmentin (amoxicillin/clavulanic acid) and cotrimoxazole in E. coli. Amin et al. also reported resistance to ampicillin and augmentin in Enterobacteriaceae (19).
Although the prevalence of MRSA infection was determined based on cefoxitin's resistance pattern, the gold standard for identifying MRSA is to detect the mecA gene (20). Getahun et al. and Nithya et al. showed a significant variation in the prevalence of MRSA ocular infections geographically at different times (21, 22). In this study, four MRSA strains and 59 MR-CoNS strains had the mecA gene. Of the 125 Staphylococcus isolates, 63 (50.4%) were carried. In other studies, the prevalence rate of MRSA was 32% among S. aureus isolates of dacryocystitis (23). Finally, the microbial profile may vary widely among geographic regions and during different periods (6). The prevalence of Gram-negative bacteria, including P. aeruginosa and Acinetobacter baumannii, and resistance to antibiotics were higher in this study than in other studies (10, 11).
5.1. Conclusions
CoNS were the most frequently isolated bacteria. The highest antibiotic susceptibility was observed to rifampin, linezolid, amikacin, and gentamicin. A high percentage of CoNS carried the mecA gene.