Abstract
Background:
Stomach disorders, including gastric cancer and gastritis, are associated with the pathogenic bacterium Helicobacter pylori. Enhanced inflammation is the characteristic of H. pylori-induced gastritis. Ligustrazine exerts anti-inflammatory properties in mouse asthma models and acute kidney injury.Objectives:
To determine the role of ligustrazine in H. pylori-induced gastritis.Methods:
Normal gastric epithelial cell line (GES-1) was cultured with H. pylori at a multiplicity of infection (MOI) of 100: 1 for 24 hours. GES-1 cell line under H. pylori condition was incubated with 100 or 200 μM ligustrazine for 24 hours. Cell viability and apoptosis were investigated by MTT and flow cytometry assays, respectively. Inflammation was assessed by determining the levels and mRNA expression of interleukins (IL)-6/8, tumor necrosis factor-α (TNF-α), and monocyte chemotactic protein 1 (MCP-1) using ELISA and qRT-PCR analysis, respectively.Results:
Helicobacter pylori infection reduced the viability and promoted the apoptosis of GES-1 cell line, accompanied by the enhanced activities of caspases 3 and 9. However, ligustrazine reversed the H. pylori-induced infection decreased viability, while increased apoptosis and caspases 3/9 activities in GES-1 cell line. Moreover, ligustrazine attenuated H. pylori-induced secretions of pro-inflammatory factors, IL-6/8, TNF-α, and MCP-1, in GES-1 cell line. The protein expression of inhibitor of NF-κB (IκBα) was downregulated in GES-1 cell line after H. pylori infection, while the protein expression levels of p65 and phosphorylation of IκBα were upregulated by H. pylori infection. On the contrary, ligustrazine decreased H. pylori-induced protein expression of IκBα, whereas increased protein expression of p65 and phosphorylation of IκBα.Conclusions:
Ligustrazine exerted protective effects on H. pylori-induced gastric epithelial cells through inhibition of gastric inflammation and apoptosis and inactivation of NF-κB pathway.Keywords
Ligustrazine Helicobacter pylori Gastritis Inflammation Apoptosis NF-κB
1. Background
Gastritis is a common disease of the digestive system in outpatient children (1). In terms of etiology, Helicobacter pylori infection is one of the most important pathogenic factors predisposing to gastritis in children (1). Helicobacter pylori, a Gram-negative bacterium, is usually acquired in childhood and colonizes at the human gastric epithelium to promote oxidative damage and secretion of pro-inflammatory factors, thus resulting in stomach disorders, including gastric cancer and gastritis (2). Eradication of H. pylori via proton pump inhibitors combined with antibiotics has been shown to reduce the rate of gastric carcinoma and relieve gastritis (2). However, the development of bacterial resistance could reduce the efficiency of antibiotic therapy (3). Therefore, other therapeutic strategies are urgently needed for the treatment of H. pylori-induced gastritis.
Through adhering to the gastric mucosa, H. pylori secrets virulent factors, such as vacuolating cytotoxin gene or cag pathogenicity island, into the epithelial cells (4-6). The virulent factors alter the cytoskeletal rearrangements and then activate NF-κB pathway to promote the secretion of interleukin 8 (IL-8), thus recruiting inflammatory cells, such as lymphocytes and neutrophils, to the infected area (4, 7). The inflammatory cells secret pro-inflammatory cytokines to cause gastric epithelial cell apoptosis and gastric mucosal injury (8). The suppression of H. pylori-induced inflammation and apoptosis has been shown to facilitate the amelioration of gastritis (9).
Ligustrazine is an alkaloid that is usually extracted from the root and stem of Chinese medicine, Ligusticum Chuanxiong Hort (10). Ligustrazine has been used as an anti-tumor agent in colon cancer (11). Moreover, ligustrazine has a wide range of pharmacological activities, including anti-oxidative stress, anti-inflammatory, anti-apoptotic, and other pharmacological effects, with high safety and fewer side effects (12). Ligustrazine protected human umbilical vein endothelial cells against hypoxia induction (13), and attenuated ovalbumin-induced inflammation in mouse asthma model (14). However, the role and mechanism of ligustrazine in gastritis are still unclear.
2. Objectives
This study investigated the role of ligustrazine in the apoptosis and inflammation of H. pylori-induced normal gastric epithelial cell line (GES-1) and then unravel the underlying mechanism.
3. Methods
3.1. Cell and Helicobacter pylori Culture
Gastric epithelial cell line-1 was purchased from Cell Resource Center of Shanghai Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium (GE Healthcare Life Sciences, Little Chalfont, UK) containing 1% streptomycin-penicillin and 10% fetal bovine serum (GE Healthcare Life Sciences) at a 37°C incubator. The medium was changed every three days. Helicobacter pylori strain NCTC11637 was purchased from Shanghai Huiying biological technology (Shanghai, China) and cultured in Columbia agar with 10% goat serum at a 37°C incubator under conditions: 85% N2, 10% CO2, and 5% O2 for 72 hours.
3.2. Cell Treatment
Gastric epithelial cell line (3 × 105 /well) was placed in the 6-well plastic plates. Helicobacter pylori was harvested from the agar plates and suspended in Dulbecco's modified Eagle's medium. The absorbance at 600 nm was adjusted to 0.6 via Microplate Reader (BioTek, Winooski, VT, USA). Helicobacter pylori at a multiplicity of infection of 100:1 was added into GES-1 cells and incubated for 24 hours.
3.3. Cell Viability and Apoptosis
After cultivation for 24 hours, GES-1 cell line under H. pylori infection was incubated with 40 µL MTT solution (2 mg/mL; Beyotime, Beijing, China) at the plastic plates for 4 hours. Following incubation with 100 µL DMSO, the absorbance at 450 nm was measured by Microplate Reader. For flow cytometry assay, GES-1 cell line was harvested following incubation with 0.25% trypsin (Thermo Fisher Scientific, Waltham, MA, USA), and then re-suspended in 100 µL binding buffer in Annexin V-FITC Apoptosis Detection kit (BD Biosciences; Franklin Lakes, NJ, USA). Fluorescein isothiocyanate Annexin V and propidium iodide were then used to stain the cells before analysis under FACS Canto II flow cytometer (BD Biosciences). The apoptotic rate was determined by FlowJo 7.6 software (Tree Star, Inc., Ashland, OR, USA).
3.4. Caspase-3 and Caspase-9 Activities
Gastric epithelial cell line under H. pylori infection was lysed with RIPA buffer (Ding Guo Chang Sheng Biotech, Beijing, China) and centrifuged at 12000 × g for 10 minutes. The supernatant was used for the detection of protein concentration using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The commercial kit (ab219915, Abcam, Cambridge, MA, USA) was used for determining the activities of caspase-3 and caspase-9.
3.5. Pro-inflammatory Cytokines Production
The supernatants of GES-1 cells were used to determine the production of IL-6, IL-8, TNF-α, and MCP-1 using ELISA kits (Abcam).
3.6. Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
TRIzol ® reagent (Thermo Fisher Scientific) was used to isolate RNAs from GES-1 cells. M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA) was used to reverse-transcribe the RNAs into cDNAs. NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) was used to detect the quality and quantity of RNAs and cDNAs. Then qRT-PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) on ViiA 7 (Applied Biosystems, Austin, TX, USA) with the following conditions: 94°C for 2 minutes; 40 cycles of 94°C for 20 seconds, 55°C for 35 seconds, 72°C for 25 seconds (15). Moreover, β-actin was used as the endogenous control. The specific primers are listed in Table 1.
Primer Sequences
| ID | Sequence (5' - 3') |
|---|---|
| β-actin | |
| F | CCTGGCACCCAGCACAAT |
| R | GCTGATCCACATCTGCTGGAA |
| IL-6 | |
| F | TGATGGATGCTTCCAAACTG |
| R | GAGCATTGGAAGTTGGGGTA |
| TNF-α | |
| F | ACTGAACTTCGGGGTGATTG |
| R | GCTTGGTGGTTTGCTACGAC |
| MCP-1 | |
| F | GATCTCAGTGCAGAGGCTCG |
| R | TTTGCTTGTCCAGGTGGTCC |
| IL-8 | |
| F | CTTGCAGCCTTCCTGATTTCT |
| R | CGCCTTTACAATAATTTCTGTGTTGGCG |
3.7. Western Blot
The supernatants of GES-1 cells (30 micrograms) were used for separating proteins by SDS-PAGE, and electro-transferred onto PVDF membranes. Primary antibodies, including anti-p65 and anti-p-p65 (1:3000; Abcam), anti-IκBα and anti-p-IκBα (1:3500; Abcam), anti-GAPDH (1:4000; Abcam), were used to probe the membranes. Following incubation with horseradish peroxidase-conjugated immunoglobulin G (1:5000; Abcam), and the blots were detected by enhanced chemiluminescence (KeyGen, Nanjing, China).
3.8. Statistical Analysis
The results from three independent experiments were presented as mean ± SD. Statistical analyses between different groups were performed with one-way analysis of variance or Student t-test under SPSS19.0 software. Values were considered significant at P < 0.05.
4. Results
4.1. Ligustrazine Attenuated Helicobacter pylori-induced Cytotoxicity in GES-1 Cell Line
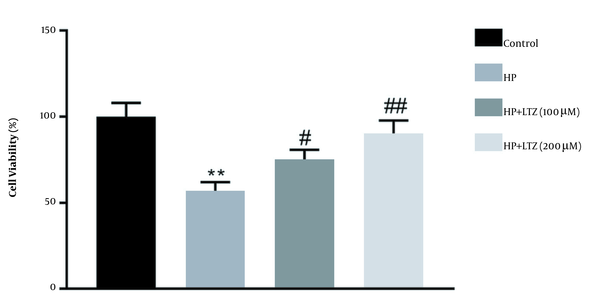
Gastric epithelial cell line was incubated with H. pylori to establish the cell model of gastritis. The result showed that H. pylori infection significantly reduced the viability of GES-1 cell line (Figure 1). Moreover, Ligustrazine treatment increased the viability of GES 1 cell line (Figure 1), which significantly attenuated H. pylori-induced cell cytotoxicity.
Ligustrazine attenuated Helicobacter pylori-induced cytotoxicity in GES-1 cell line. Ligustrazine treatment increased viability of GES-1 cell line during H. pylori-induced infection. ** Vs. control, P < 0.01. #, ## Vs. H. pylori, P < 0.05, P < 0.01.
4.2. Ligustrazine Attenuated Helicobacter pylori-induced Inflammation in GES-1 Cell Line
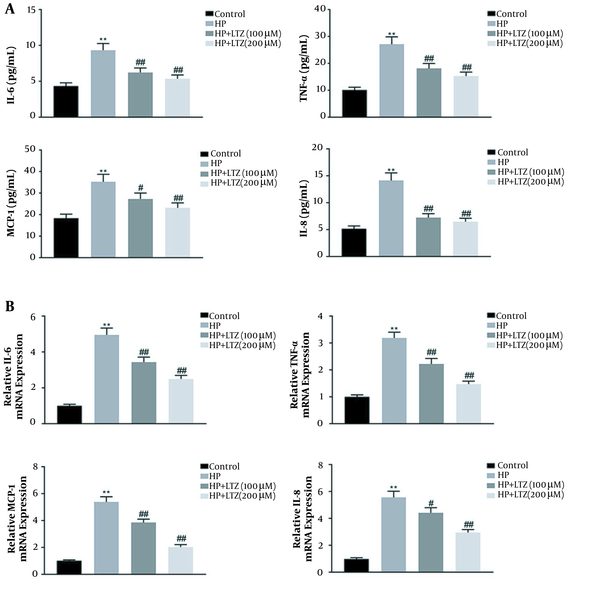
ELISA assays demonstrated that H. pylori infection upregulated the secretion of pro-inflammatory cytokines, including IL-6/8, TNF-α, and MCP-1, in GES-1 cell line (Figure 2A). However, ligustrazine treatment reduced H. pylori-induced levels of IL-6/8, TNF-α, and MCP-1 in GES-1 cell line (Figure 2A). Moreover, ligustrazine also attenuated mRNA expression of H. pylori-induced of IL-6/8, TNF-α, and MCP-1 in GES-1 cell line (Figure 2B), suggesting the anti-inflammatory effect of ligustrazine against H. pylori-induced gastritis.
Ligustrazine attenuated Helicobacter pylori-induced inflammation in GES-1 cell line. (A) Ligustrazine treatment attenuated H. pylori-induced levels of IL-6/8, TNF-α, and MCP-1 in GES-1 cell line. (B) Ligustrazine treatment attenuated H. pylori-induced mRNA expression levels of IL-6/8, TNF-α, and MCP-1 in GES-1 cell line. ** Vs. control, P < 0.01. #, ## Vs. H. pylori, P < 0.05, P < 0.01.
4.3. Ligustrazine Attenuated Helicobacter pylori-induced Apoptosis in GES-1 Cell Line
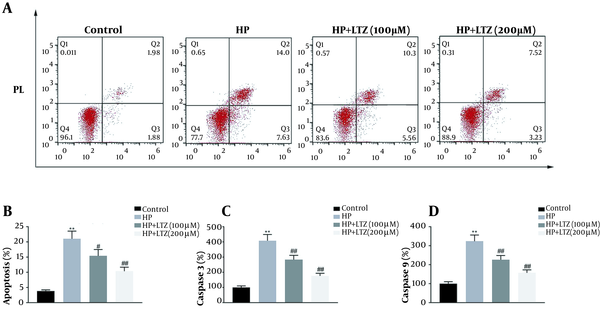
In addition to the anti-inflammatory effects of ligustrazine, its anti-apoptotic effect on GES-1 cell line was subsequently assessed. Flow cytometry assay demonstrated that H. pylori infection promoted the apoptosis of GES-1 cell line (Figure 3A and B). However, ligustrazine treatment decreased H. pylori-induced apoptotic cell number (Figure 3A and B). Moreover, ligustrazine also attenuated H. pylori-induced activities of caspase-3 (Figure 3C) and caspase-9 (Figure 3D) in GES-1 cell line, suggesting the anti-apoptotic effect of ligustrazine against H. pylori-induced gastritis.
Ligustrazine attenuated Helicobacter pylori-induced apoptosis in GES-1 cell line. (A) Ligustrazine treatment attenuated H. pylori-induced apoptotic rate of GES-1 cell line, as shown by flow cytometry assay. (B) Statistical results according to the results of (A). (C) Ligustrazine treatment attenuated H. pylori-induced activity of caspase-3 in GES-1 cell line. (D) Ligustrazine treatment attenuated H. pylori-induced activity of caspase-9 in GES-1 cell line. ** Vs. control, p < 0.01. #, ## Vs. H. pylori, P < 0.05, P < 0.01.
4.4. Ligustrazine Attenuated Helicobacter pylori-induced NF-κB Activation in GES-1 Cell Line
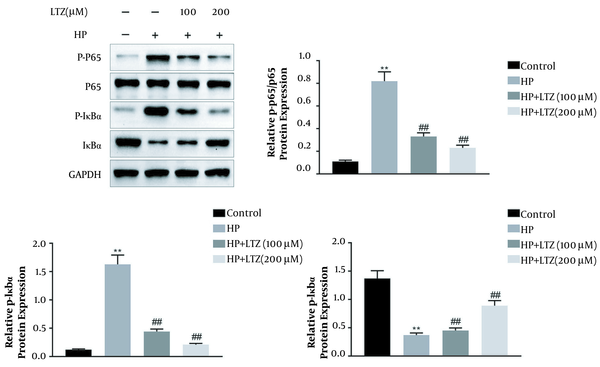
Although the protein expression of p65 was not affected by H. pylori infection in GES-1 cell line (Figure 4), the protein expression of the phosphorylation of p65 was enhanced in GES-1 cell line after H. pylori infection (Figure 4). Moreover, the expression of the inhibitor of NF-κB (IκBα) was reduced and the expression of the phosphorylation of IκBα was enhanced in H. pylori-infected GES-1 cell line (Figure 4), indicating that H. pylori infection promoted the activation of NF-κB in GES-1 cell line. Ligustrazine reversed the effects of H. pylori infection on the protein expression of IκBα, IκBα, and p65 phosphorylation in GES-1 cell line (Figure 4), revealing that ligustrazine attenuated H. pylori-induced NF-κB activation in GES-1 cell line.
Ligustrazine attenuated Helicobacter pylori-induced NF-κB activation in GES-1 cell line. Ligustrazine treatment decreased H. pylori-induced protein expression of IκBα, and increased protein expression of the phosphorylation of p65 in GES-1 cell line. ** Vs. control, P < 0.01. ## Vs. H. pylori, P < 0.01.
5. Discussion
Ligusticum chuanxiong Hort has been widely used as an effective medical plant (16), and the aqueous extract of L. chuanxiong Hort showed anti-H. pylori capacity (16). Ligusticum chuanxiong Hort was used for the prevention of ulcer diseases (17). Since ligustrazine was a bioactive component isolated from L. chuanxiong Hort (18), with potential anti-inflammatory, antioxidant, and anti-fibrosis effects, its exact effects on H. pylori-induced gastritis was evaluated in this study.
Infiltration of inflammatory cells into gastric mucosa was generally associated with H. pylori infection, and the secretion of pro-inflammatory cytokines, proteolytic enzymes, and reactive oxygen species by these inflammatory cells could result in mucosal damage (19). For example, IL-6, MCP-1, and TNF-α levels were upregulated by H. pylori infection in GES-1 cells (20). In the present study, H. pylori infection enhanced the mRNA and protein expression levels of IL-6/8, MCP-1, and TNF-α in GES-1 cells, which were consistent with a previous study, showing that ligustrazine reduced inflammation in the kidney cells post-ischemia/reperfusion injury with decreased levels of IL-6, MCP-1, and TNF-α (21). Besides, our results also confirmed that ligustrazine attenuated H. pylori-induced increased levels of IL-6/8, MCP-1, and TNF-α in GES-1 cell line, suggesting its anti-inflammatory effect on H. pylori-induced gastritis.
Helicobacter pylori has also been shown to promote the apoptosis of gastric epithelial cells through inducible caspase-3/E-cadherin pathway (22) or caspase-3/9 activation (23). In this study, we also noted the increased apoptosis and caspase-3/9 activities in GES-1 cell line caused by H. pylori. In H. pylori-infected children, resolution of gastritis and eradication of the bacterium restored the apoptosis of gastric epithelial cells to baseline levels (24), suggesting that anti-apoptotic agents might be an effective therapeutic strategy for the prevention of H. pylori-induced stomach disorders. Ligustrazine has been shown to protect against homocysteine-induced cell apoptosis through modulation of mitochondrial dysfunction (25). Results in this study demonstrated that ligustrazine attenuated H. pylori-induced increased apoptosis and caspase-3/9 activities in GES-1 cell line, suggesting its anti-apoptotic effect against H. pylori-induced gastritis.
Activation of NF-κB pathway in gastric epithelial cells is a marker of chronic inflammation and gastric cancer induced by H. pylori (26). NF-κB is generally sequestered in the cytoplasm, and translocate into the nucleus through activation and phosphorylation (27). The activation of NF-κB is mediated by the inhibitory proteins IκB, and phosphorylation of IκB promotes self-ubiquitination and proteasome-mediated degradation, thus liberating nuclear translocation of NF-κB to activate target genes, including IL-8 (4). Suppression of NF-κB activation was involved in genistein-repressed gastric inflammation and apoptosis in H. pylori-induced rats (9). Here, IκBα was reduced, IκBα and phosphorylation of p65 were enhanced in GES-1 post-H. pylori infection. Ligustrazine upregulated IκBα, while downregulated phosphorylation of p65 in H. pylori-induced GES-1 cell line, suggesting that ligustrazine exerted gastroprotective effects on gastric epithelial cells through inactivation of NF-κB pathway.
5.1. Conclusions
Ligustrazine counteracted the promotive effects of H. pylori on the levels of pro-inflammatory cytokines (IL-6/8, MCP-1, and TNF-α) and gastric epithelial cell apoptosis through suppression of NF-κB activation. However, the in vivo effect of ligustrazine on H. pylori-induced rats should be performed to further confirm the therapeutic effects of ligustrazine on H. pylori-induced gastric disorders.
References
-
1.
Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50(6):657-67. [PubMed ID: 25901896]. [PubMed Central ID: PMC4673514]. https://doi.org/10.3109/00365521.2015.1019918.
-
2.
Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196-202. [PubMed ID: 23981572]. https://doi.org/10.1016/j.canlet.2013.08.016.
-
3.
O'Connor A, Lamarque D, Gisbert JP, O'Morain C. Treatment of Helicobacter pylori infection 2017. Helicobacter. 2017;22(Suppl 1). e12410. [PubMed ID: 28891137]. https://doi.org/10.1111/hel.12410.
-
4.
Waskito LA, Salama NR, Yamaoka Y. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2018;23(Suppl 1). e12516. [PubMed ID: 30203582]. https://doi.org/10.1111/hel.12516.
-
5.
Mousavi S, Dehkordi FS, Rahimi E. Virulence factors and antibiotic resistance of Helicobacter pylori isolated from raw milk and unpasteurized dairy products in Iran. J Venom Anim Toxins Incl Trop Dis. 2014;20:51. [PubMed ID: 25873940]. [PubMed Central ID: PMC4396062]. https://doi.org/10.1186/1678-9199-20-51.
-
6.
Ranjbar R, Farsani FY, Dehkordi FS. Phenotypic analysis of antibiotic resistance and genotypic study of the vacA, cagA, iceA, oipA and babA genotypes of the Helicobacter pylori strains isolated from raw milk. Antimicrob Resist Infect Control. 2018;7:115. [PubMed ID: 30288255]. [PubMed Central ID: PMC6162967]. https://doi.org/10.1186/s13756-018-0409-y.
-
7.
Ranjbar R, Yadollahi Farsani F, Safarpoor Dehkordi F. Antimicrobial resistance and genotyping ofvacA,cagA, andiceAalleles of theHelicobacter pyloristrains isolated from traditional dairy products. J. Food Saf. 2018;39(2). https://doi.org/10.1111/jfs.12594.
-
8.
Arismendi Sosa AC, Salinas Ibanez AG, Perez Chaca MV, Penissi AB, Gomez NN, Vega AE. Inflammatory response induced by Helicobacter pylori infection in lung. Microb Pathog. 2020;142:104103. [PubMed ID: 32112810]. https://doi.org/10.1016/j.micpath.2020.104103.
-
9.
Siriviriyakul P, Werawatganon D, Phetnoo N, Somanawat K, Chatsuwan T, Klaikeaw N, et al. Genistein attenuated gastric inflammation and apoptosis in Helicobacter pylori-induced gastropathy in rats. BMC Gastroenterol. 2020;20(1):410. [PubMed ID: 33297977]. [PubMed Central ID: PMC7724785]. https://doi.org/10.1186/s12876-020-01555-x.
-
10.
Zhao T, Fu Y, Sun H, Liu X. Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life. 2018;70(1):60-70. [PubMed ID: 29247598]. https://doi.org/10.1002/iub.1704.
-
11.
Chen X, Yang X, Mu J, Yue C. Ligustrazine inhibits the viability and motility of colon cancer cells. Transl Cancer Res. 2020;9(5):3203-13. https://doi.org/10.21037/tcr-20-940.
-
12.
Su Q, Lv X, Ye Z. Ligustrazine attenuates myocardial injury induced by coronary microembolization in rats by activating the PI3K/AKT pathway. Oxid Med Cell Longev. 2019;2019:6791457. [PubMed ID: 31191802]. [PubMed Central ID: PMC6525935]. https://doi.org/10.1155/2019/6791457.
-
13.
Wei S, Wang H. Ligustrazine promoted hypoxia-treated cell growth by upregulation of miR-135b in human umbilical vein endothelial cells. Exp Mol Pathol. 2019;106:102-8. [PubMed ID: 30576641]. https://doi.org/10.1016/j.yexmp.2018.12.005.
-
14.
Wei Y, Liu J, Zhang H, Du X, Luo Q, Sun J, et al. Ligustrazine attenuates inflammation and the associated chemokines and receptors in ovalbumine-induced mouse asthma model. Environ Toxicol Pharmacol. 2016;46:55-61. [PubMed ID: 27438894]. https://doi.org/10.1016/j.etap.2016.07.005.
-
15.
Sork H, Conceicao M, Corso G, Nordin J, Lee YXF, Krjutskov K, et al. Profiling of extracellular small RNAs highlights a strong bias towards non-vesicular secretion. Cells. 2021;10(6). [PubMed ID: 34207405]. [PubMed Central ID: PMC8235078]. https://doi.org/10.3390/cells10061543.
-
16.
Ran X, Ma L, Peng C, Zhang H, Qin LP. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm Biol. 2011;49(11):1180-9. [PubMed ID: 22014266]. https://doi.org/10.3109/13880209.2011.576346.
-
17.
Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol. 2005;98(3):329-33. [PubMed ID: 15814268]. https://doi.org/10.1016/j.jep.2005.01.020.
-
18.
Xiong L, Fang ZY, Tao XN, Bai M, Feng G. Effect and mechanism of ligustrazine on Th1/Th2 cytokines in a rat asthma model. Am J Chin Med. 2007;35(6):1011-20. [PubMed ID: 18186587]. https://doi.org/10.1142/S0192415X07005478.
-
19.
Israel DA, Peek RM. pathogenesis of Helicobacter pylori-induced gastric inflammation. Aliment Pharmacol Ther. 2001;15(9):1271-90. [PubMed ID: 11552897]. https://doi.org/10.1046/j.1365-2036.2001.01052.x.
-
20.
Lian DW, Xu YF, Ren WK, Fu LJ, Chen FJ, Tang LY, et al. Unraveling the novel protective effect of patchouli alcohol against helicobacter pylori-induced gastritis: Insights into the molecular mechanism in vitro and in vivo. Front Pharmacol. 2018;9:1347. [PubMed ID: 30524287]. [PubMed Central ID: PMC6262355]. https://doi.org/10.3389/fphar.2018.01347.
-
21.
Jiang G, Xin R, Yuan W, Zhang L, Meng X, Sun W, et al. Ligustrazine ameliorates acute kidney injury through downregulation of NOD2‑mediated inflammation. Int J Mol Med. 2020;45(3):731-42. https://doi.org/10.3892/ijmm.2020.4464.
-
22.
Yang Y, Du J, Liu F, Wang X, Li X, Li Y. Role of caspase-3/E-cadherin in helicobacter pylori-induced apoptosis of gastric epithelial cells. Oncotarget. 2017;8(35):59204-16. [PubMed ID: 28938629]. [PubMed Central ID: PMC5601725]. https://doi.org/10.18632/oncotarget.19471.
-
23.
Shibayama K, Doi Y, Shibata N, Yagi T, Nada T, Iinuma Y, et al. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect Immun. 2001;69(5):3181-9. [PubMed ID: 11292739]. [PubMed Central ID: PMC98275]. https://doi.org/10.1128/IAI.69.5.3181-3189.2001.
-
24.
Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151(6):1695-703. [PubMed ID: 9403720]. [PubMed Central ID: PMC1858359].
-
25.
Fan X, Wang E, He J, Zhang L, Zeng X, Gui Y, et al. Ligustrazine protects homocysteine-induced apoptosis in human umbilical vein endothelial cells by modulating mitochondrial dysfunction. J Cardiovasc Transl Res. 2019;12(6):591-9. [PubMed ID: 31359360]. https://doi.org/10.1007/s12265-019-09900-6.
-
26.
Bontems P, Aksoy E, Burette A, Segers V, Deprez C, Mascart F, et al. NF-kappaB activation and severity of gastritis in Helicobacter pylori-infected children and adults. Helicobacter. 2014;19(3):157-67. [PubMed ID: 24661597]. https://doi.org/10.1111/hel.12118.
-
27.
Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29(10):469-78. [PubMed ID: 18775672]. [PubMed Central ID: PMC5751948]. https://doi.org/10.1016/j.it.2008.07.003.