1. Introduction
Ralstonia pickettii, an aerobic Gram-negative bacterium, is found in water and soil. This bacterium has growingly increased the incidence of infection (1). It can cause opportunistic infections in immunocompromised patient. Risk factors for R. pickettii infection include cancer surgery, organ transplant, chemotherapy, poor immunity, and in situ blood vessel catheterization (2). In this study, we reported a case of R. pickettii in a woman with normal immunity. To our knowledge, this is the first report of sepsis caused by R. pickettii in the absence of risk factors. We described the infection diagnosis while also raising awareness about this type of infection.
2. Case Presentation
On April 2, 2021, a 36-year-old woman with a thyroid nodule underwent thyroid surgery in the Second Affiliated Hospital of the Guangxi Medical University (Nanning, China). The patient was 160 cm in height and 50 kg in weight, and she worked in a sole proprietorship. The patient’s parents and children were in good health, and none of them had a similar medical history. The patient had no medication history before admission. She lacked relevant risk factors for opportunistic pathogen infections and received no special preoperative laboratory examination. The patient developed a fever, and her temperature rose to 40°C after surgery. She also exhibited shock (e.g., elevated heart rate, reduced blood pressure, and decreased urine output). The clinical signs and symptoms of the patient were highly similar to those observed in hyperthyroidism.
When her clinical signs became severe, she was transferred to the intensive care unit. Blood analysis revealed a white blood cell (WBC) count of 1.47 × 109/L, neutrophilia (83.6%), a C reactive protein (CRP) level of 272 mg/L, and a procalcitonin (PCT) level exceeding 100 ng/mL. Considering the possibility of an invasive infection, we obtained two blood cultures before starting antibiotic therapy (imipenem and daptomycin) on April 2, 2021 (9:00 pm). Gram staining after 10 h revealed Gram-negative rods in the aerobic culture obtained from peripheral blood drawn from the inner left and right elbow veins. After 1 day, domed, smooth colonies grew on blood agar plates, and non-lactose-fermenter colonies grew on MacConkey agar plates. The matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) identified R. mannitolilytica (confidence value, 2.319), but subsequent 16S rRNA gene sequencing identified R. pickettii.
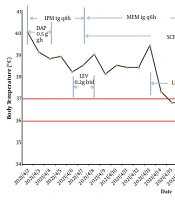
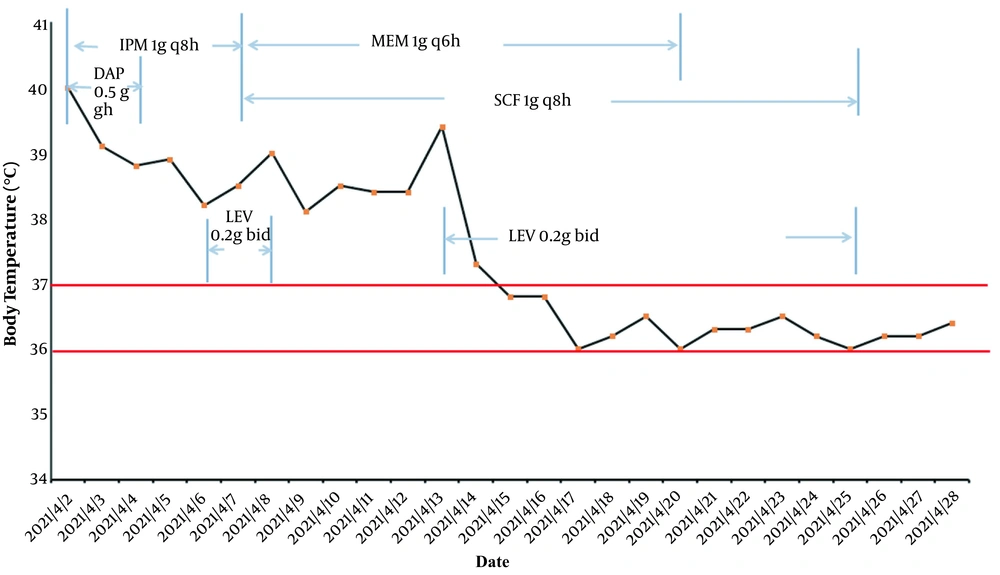
On April 10, 2021, the cultured thyroid drainage fluid and the tip of the thyroid drainage tube also tested positive for R. pickettii, a finding related to bacterial contamination in blood. Drug susceptibility tests were performed using the BD PhoenixTM-100 Automated Microbiology System. The results were analyzed according to instructions from the Clinical and Laboratory Standards Institute (CLSI 2020), as presented in Table 1. The bacterium was sensitive to ciprofloxacin (CIP), cefoperazone/sulbactam (SCF), sulfamethoxazole (SXT), and ceftriaxone (CRO). Following the drug susceptibility test results, doctors in the intensive care unit then prescribed levofloxacin (LEV). According to the inflammatory index [e.g., WBC, PCT, and CRP (data not shown)] and the patient’s body temperature, the doctor changed the antibacterial drugs several times (Figure 1). Since the patient had sepsis, her liver function was severely compromised, and treatments, such as liver protection and nutritional support, were provided. The patient was eventually discharged on day 27 in good clinical condition.
| Antimicrobial Drug | Minimal Inhibitory Concentration (µg/mL) | |
|---|---|---|
| Blood | Drainage Fluid | |
| Amikacin (AK) | > 32 (R) | > 32 (R) |
| Ciprofloxacin (CIP) | ≤ 0.5 (S) | ≤ 0.5 (S) |
| Tobramycin (TM) | > 8 (R) | > 8 (R) |
| Meropenem (MEM) | > 8 (R) | > 8 (R) |
| Gentamicin (GM) | > 2 (R) | > 2 (R) |
| Cefoperazon/sulbactam (SCF) | ≤ 0.5/8 (S) | ≤ 0.5/8 (S) |
| Ceftazidime (CAZ) | > 32 (R) | > 32 (R) |
| Aztreonam (ATM) | > 32 (R) | > 32 (R) |
| Levofloxacin (LEV) | ≤ 1 (S) | ≤ 1 (S) |
| Sulfamethoxazole (SXT) | ≤ 1/19 (S) | ≤ 1/19 (S) |
| Piperacillin/Tazobactam (TZP) | > 64/4 (R) | > 64/4 (R) |
| Imipenem (IPM) | > 8 (R) | > 8 (R) |
| Chloramphenicol (C) | > 16 (R) | > 16 (R) |
| Cefepime (FEP) | 16 (I) | 16 (I) |
| Ceftriaxone (CRO) | 2 (S) | 2 (S) |
Abbreviations: I, intermediate; R, resistant; S, sensitive.
The surgery room environment, parental solutions, and airway tubes used for anesthesia were sampled and tested via culture for 7 days to avoid further possible outbreaks and determine the source of infection, and the test results were negative.
3. Discussion
Ralstonia, a genus of aerobic Gram-negative, non-fermenting bacteria, is considered an opportunistic pathogen. Three Ralstonia species (R. pickettii, R. insidiosa, and R. mannitolilytica) are clinically significant (3). Contaminated solutions and water are considered the sources of nosocomial R. pickettii infection in medical settings. Ralstonia pickettii has been recovered from various clinical specimens (e.g., blood, urine, and cerebrospinal fluid). We conducted a comprehensive literature review to determine circumstances under which Ralstonia was linked to bacteremia. In 33 reported cases, co-morbidities were described (2, 4-7). Contrastingly, our patient was a 36-year-old woman with no known history of underlying chronic disease or immunosuppression. The patient’s thyroid surgery was considered a clear surgery according to the surgical incision classification. She had no obvious risk factors. Nevertheless, her clinical signs suggested a thyroid crisis when the patient experienced a high fever of sudden onset after surgery. To avoid the possibility of a missed infection, we quickly obtained blood cultures for monitoring, enabling the causative agent of bloodstream infection to be identified only 10 h after surgery. This proved crucial for the choice of antibacterial treatment and the patient's final recovery. This also emphasized the importance of blood culture testing when a hospitalized patient has an unexplained high fever (8).
One limitation of the current case report was that the route of infection by the opportunistic pathogenic was not clarified. According to culture testing, the surgery room environment, parental solutions, and airway tubes were negative for contamination. Cultures of blood, thyroid drainage fluid, and the tip of the thyroid drainage tube tested positive for R. pickettii, in line with the result of in vitro drug susceptibility testing (Table 1). Given that thyroid drainage fluid and the tip of the thyroid drainage tube tested positive after the results of blood culture and bacterial gene sequencing of thyroid tissue were negative (data not shown), we considered that the positive results of the fluid and the tube were related to bacterial contamination in blood, as opposed to the thyroid tissue contamination. MALDI-TOF MS can improve the speed at which unusual pathogens are identified. The bacterium was initially identified as R. mannitolilytica by MALDI-TOF MS. However, subsequent 16S rRNA gene sequencing identified the infecting pathogen as R. pickettii, with the confidence value of 2.319. The Bruker Biotyper software was used to analyze blood culture specimens, and the estimated sensitivity with a cut-off of ≥ 2.0 was 74.6%, with an estimated specificity of 88.0% (9). Therefore, 16S rRNA sequencing remains a reliable identification system (10).
Here, R. pickettii was for the first time identified as a cause of bloodstream infection in our hospital. Ralstonia species display widespread antimicrobial resistance to ampicillin, meropenem, and aminoglycoside antibiotics. The bacterium isolated in this case was only sensitive to CIP, SCF, LEV, SXT, and CRO, a finding consistent with the drug resistance characteristics reported in other studies (11). By reviewing the history of antimicrobial treatment (Figure 1), LEV was identified as an effective drug for the patient, who was eventually discharged. This is consistent with in vitro antibacterial drug susceptibility test results. This emphasizes that in vitro drug susceptibility testing is useful and necessary to guide the clinical use of antibacterial drugs. The cause of its widespread resistance may be related to two variants of the class D β-lactamase-encoding gene blaOXA (blaOXA-573 and blaOXA-574) (5). However, its mechanism should be further studied in depth.
3.1. Conclusions
We described a case of bloodstream infection caused by R. pickettii in a young woman with normal immunity, and the typing of the microbe was performed in our laboratory. Clinicians should be aware that opportunistic pathogens may cause high fever after thyroid surgery. Therefore, in addition to post-surgery thyroid crisis, an infection should be considered in patients with pyrexia. Finally, drug resistance is rare in bacterial species such as R. pickettii. The mechanism of drug resistance should be studied in depth.