Dear Editor,
Diphtheria is still a health problem in Indonesia. The World Health Organization (WHO) data show that Indonesia is one of the countries with the highest diphtheria rates (1). The causative agents of diphtheria (Corynebacterium diphtheriae, C. ulcerans, and C. pseudotuberculosis) can produce the diphtheria toxin (2-4). The ability of bacteria to synthesize the diphtheria toxin (bacterial toxigenicity) can be examined in the laboratory. The Elek test has been recognized by the WHO and is commonly used worldwide to examine bacterial toxigenicity. However, the Elek test needs specific basic reagents that are not always available in microbiology laboratories (5, 6). Herein, we evaluated the use of Columbia blood agar base (CBAB) as an alternative reagent for the Elek test. Columbia blood agar base is a reagent that is easily available in microbiology laboratories.
We examined 30 bacterial isolates consisting of diphtheria-causing bacteria and non-diphtheria-causing bacteria, both reference, and clinical isolates. The reference isolates of the diphtheria-causing bacteria consisted of two toxigenic C. diphtheriae (NCTC 10648 and NCTC 3984), nontoxigenic C. diphtheriae (NCTC 10356), and nontoxigenic C. ulcerans (NCTC 12077); the non-diphtheria-causing bacteria consisted of C. striatum (NCTC 764), C. minutissimum (ATCC 23346), C. pseudodiphthericum (ATCC 10700), Klebsiella pneumoniae (ATCC BAA-1144), Staphylococcus aureus (ATCC 12493), and S. pneumoniae (ATCC 10015). The clinical isolates of the diphtheria-causing bacteria consisted of 19 toxigenic C. diphtheriae, one nontoxigenic C. diphtheriae, and no clinical isolates of non-diphtheria-causing bacteria. The toxigenic and nontoxigenic strains were used to ensure the accuracy of the examination and avoid false positives and false negatives. Several non-diphtheria-causing bacteria were used to ensure that there was no cross-reaction with other metabolites or toxins produced by these bacteria.
The standard method of the Elek test developed by Elek (7) with a modification was conducted by Engler (6) and used as a control. A total of 20 g Proteose Peptone No. 2 (Difco) was dissolved in 500 mL of distilled water, and then we added 3.25 mL of NaOH 40% and heated the solution until it dissolved completely. Next, the solution was filtered using a Whatman glass filter (GF/F grade) in order to remove phosphate precipitates. Then, 0.7 mL lactic acid 90% was added to the solution, followed by mixing with 3.0 g maltose, and the pH was adjusted to 7.8. The second medium was prepared by dissolving 5 g of NaCl and 10 g of Bacteriology Agar into 500 mL of distilled water and heated until the solution dissolved completely. Next, the pH was set to 7.8. Both types of medium mixtures were poured into the tubes (2.5 mL per tube) and sterilized at 121°C for 15 minutes, and stored in the refrigerator. This was called the Elek base medium.
The Elek test was conducted by melting a tube of Elek base medium until it melted completely, and then we let it stand at approximately 56°C. Gently, 0.5 mL of Newborn Calf Serum was supplemented, mixed, and poured into 4.5-inch glass plates, and we let it stand until it hardened completely. The ADS disc was placed in the center of the plate, and the bacteria isolates were inoculated at a distance of 1 cm from the edge of the ADS disc (Figure 1). The plates were incubated at 35 - 37°C for 24 - 48 hours. Finally, the Elek test result was assessed by observing the formation of a white transverse precipitation line between the ADS and the bacterial inoculation site. If a precipitation line appeared, it was concluded that the isolate was toxigenic; otherwise, if there was no precipitation line, it was considered nontoxigenic (6, 8).
Comparison of the results of the Elek test using the standard method (left side) and Columbia blood agar base (right side): high toxin positive control (++), weak toxin positive control (+), negative control (-), toxigenic samples (A and C), nontoxigenic sample (B).
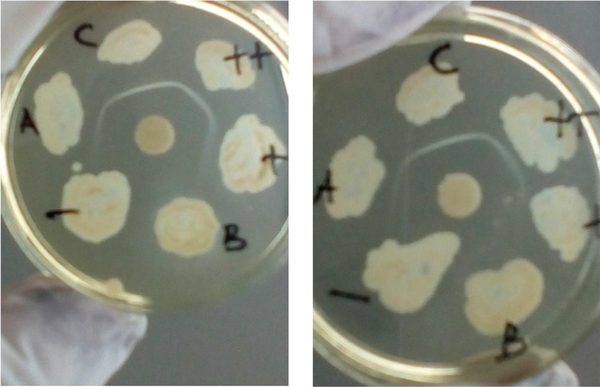
We used CBAB to substitute the Elek base medium, and N-Z Amine A (optional) was added to enhance diphtheria toxin production (8, 9). Then, 39 g of Columbia Blood Agar Base and 30 g of N-Z Amine A were dissolved in 1000 mL distilled water, heated until the solution dissolved evenly, then poured into the tubes (2.5 mL per tube) and sterilized at 121°C for 15 minutes and stored in the refrigerator. The Elek test procedures, including the use of newborn bovine (calf) serum and diphtheria antitoxin (ADS), were similar to those used in the standard method (6). The results of the identification of bacterial toxigenicity using the standard Elek test and CBAB can be seen in Figure 1. The toxigenicity of the diphtheria-causing bacteria and non-diphtheria-causing bacteria were identified correctly by both the standard method and CBAB. The results showed that 21 isolates were identified as toxigenic, while the others were nontoxigenic. The quality of the white lines as the toxigenicity markers was similar in both methods. We concluded that CBAB could be used as an alternative medium to identify the toxigenicity of diphtheria-causing bacteria with the Elek test. We also thank Sunarno for the advice in the research and in the writing of this paper.
References
-
1.
World Health Organization. Diphtheria reported cases. Geneva, Switzerland: World Health Organization; 2020.
-
2.
Meinel DM, Kuehl R, Zbinden R, Boskova V, Garzoni C, Fadini D, et al. Outbreak investigation for toxigenic Corynebacterium diphtheriae wound infections in refugees from Northeast Africa and Syria in Switzerland and Germany by whole genome sequencing. Clin Microbiol Infect. 2016;22(12):1003 e1-8. [PubMed ID: 27585943]. https://doi.org/10.1016/j.cmi.2016.08.010.
-
3.
Wake K, Kikuchi J, Uchida M, Nemoto M, Kaji Y, Yokoyama T, et al. Transmission of toxigenic Corynebacterium ulcerans infection with airway obstruction from cats to a human. Acute Med Surg. 2021;8(1). e705. [PubMed ID: 34804555]. [PubMed Central ID: PMC8588950]. https://doi.org/10.1002/ams2.705.
-
4.
Selim SA, Mohamed FH, Hessain AM, Moussa IM. Immunological characterization of diphtheria toxin recovered from Corynebacterium pseudotuberculosis. Saudi J Biol Sci. 2016;23(2):282-7. [PubMed ID: 26981011]. [PubMed Central ID: PMC4778578]. https://doi.org/10.1016/j.sjbs.2015.11.004.
-
5.
Neal SE, Efstratiou A, International Diphtheria Reference L; Dipnet. International external quality assurance for laboratory diagnosis of diphtheria. J Clin Microbiol. 2009;47(12):4037-42. [PubMed ID: 19828749]. [PubMed Central ID: PMC2786670]. https://doi.org/10.1128/JCM.00473-09.
-
6.
Engler KH, Glushkevich T, Mazurova IK, George RC, Efstratiou A. A modified Elek test for detection of toxigenic corynebacteria in the diagnostic laboratory. J Clin Microbiol. 1997;35(2):495-8. [PubMed ID: 9003626]. [PubMed Central ID: PMC229610]. https://doi.org/10.1128/jcm.35.2.495-498.1997.
-
7.
Elek SD. The plate virulence test for diphtheria. J Clin Pathol. 1949;2(4):250-8. [PubMed ID: 15396422]. [PubMed Central ID: PMC1023322]. https://doi.org/10.1136/jcp.2.4.250.
-
8.
Fitriana F, Sunarno S, Syarif AK, Karyana M, Rosana Y, Moehario LH. A new modified medium for Simultaneous Cystinase and elek tests of bacteria causing diphtheria. Bali Med J. 2019;8(1):334. https://doi.org/10.15562/bmj.v8i1.1231.
-
9.
Stainer DW, Corkill JM, Scholte MJ. Preparation and properties of diphtheria toxoids in submerged culture. 3. Development of a new semisynthetic medium. Can J Microbiol. 1968;14(10):1155-60. [PubMed ID: 5681528]. https://doi.org/10.1139/m68-193.