Abstract
Background:
Brucellosis is one of the main causes of economic loss in domestic animals due to abortions and infertility. Brucella is a facultative intracellular pathogen that survives in other cell types in addition to phagocytes. T cell mediated responses are necessary to eradicate the infection due to Brucella.Objectives:
In this study the potential of recombinant protein rTF/Bp26/Omp31 as a novel Brucella subunit vaccine, protective efficacy, and immune response was evaluated in BALB/c mice.Methods:
After in silico design of rTF/Bp26/Omp31 structure, the gene was cloned and expressed in Escherichia coli BL21 (DE3). Finally, purified protein by Ni-NTA was used as immunogenic to immunized mice.Results:
Mice immunized with rTF/Bp26/Omp31 showed a vigorous humoral and cellular mediated immunity; results were compatible with IgG1 and IgG2a levels as well as high amounts of IFN-γ, IL-12, IL-4 and IL-10 production in immunized mice, compared with control groups (P < 0.05). Compared to control groups, mice vaccinated with rTF/Bp26/Omp31 showed a significant response and protection against subsequent Brucella infection (P < 0.05).Conclusions:
Statistical analyses indicate similar responses in immunized mice exposed to rTF/Bp26/Omp31, compared with B. abortus RB51 and B. melitensis Rev.1 vaccines. These results showed the potential of rTF/Bp26/Omp31 as a candidate for the development of a subunit vaccine against brucellosis.Keywords
Vaccine Recombinant Omp31 Protein Trigger Factor (TF) Bp26 Protein Brucella
1. Background
Brucellosis is an important zoonotic infection that leads to health problems and economic consequences in many countries (1). This infection occurs through ingestion or inhalation of Brucella via the oral and nasal cavities (1-3). Infection is characterized by abortion and infertility in many domestic animals as well as chronic infections and inflammation in humans (4). The most important way to infection control and prevention against brucellosis in animals is performed by administration of the live attenuated vaccine of Brucella strains, such as: Brucella abortus RB51, B. abortus strain S19 and B. melitensis strain Rev.1 (5); and a human Brucella vaccine does not exist (6). Despite the efficacy of live attenuated vaccine, these vaccines have some disadvantages: the ability to cause disease in humans, abortion in pregnant animals and difficulty in the diagnostic validation of infection stages in vaccinated animals (5, 7). Because of the problems derived from the utilization of attenuated and killed vaccines in humans and animals, the development of a beneficial subunit vaccine against brucellosis is desirable (7-9).
Recombinant subunit vaccines have predetermined compositions with suitable homogeneity; they can be controlled to ensure good production and are completely inert (5, 7, 10). Recently, intracellular and cell surface components of Brucella spp. have been considered as protective antigens, but only a few antigenic components have suitable immunogenic activity (6, 8, 11-13) including: Brucella lumazine synthase-BLS, ribosomal protein L7/L12, sugar-binding 39-kDa protein-p39, periplasmic immunogenic protein-Bp26, molecular chaperone-DnaK, outer membrane protein-Omp16,19,25 and 31, Cu/Zn superoxide dismutase-SodC, SurA Peptidyl-prolyl cis-trans isomerase and Trigger factor (Tig)/TF (5, 10-12, 14, 15). Despite the immunogenicity of these antigens, the desirable protection against bacteria could be improved using a multiple subunit vaccine.
Combination of such antigens in subunit-vaccine formulation can potentially augment the protective efficacy of each antigen alone (6, 8, 9, 15). TF (16-18), Bp26 (19-21), and Omp31 (22-24) have been characterized as potential immunogenic antigens and have been previously studied to determine their protective immunogenicity. Omp31 is involved in infection pathogenesis as a virulence factor; in appropriate condition of cell-mediated immunity, Omp31 could play a protective role against Brucella (22, 24, 25). Highly conserved periplasmic Bp26 protein of Brucella spp. is an important antigen with particular adjuvant and protective activity that can induce humoral and cellular responses (19, 21, 26, 27). Trigger factor/Tig (TF)-immunophilin is a cytoplasmic protein with immunogenicity potential, which is involved in pathogenesis of brucellosis (16-18).
2. Objectives
In this study, we developed a novel structure containing TF, Bp26, and Omp31 putative antigenic determinants of Brucella to evaluate the protective efficacy and immune response of this recombinant protein against Brucella in the murine model.
3. Methods
3.1. Ethics Statement
All experimental procedures and animal care were performed in compliance with the institutional animal care guidelines of the ethics committee of Kerman University of Medical Sciences (Ethical Approval Code-K/93/193, 9.8.2014).
3.2. Bacterial Strains
The live attenuated B. abortus RB51 and B. melitensis Rev1 vaccine strains were obtained from Razi Vaccine and Serum Research Institute, Karaj, Iran. Brucella abortus strain 544 and B. melitensis 16M were obtained from the Microbial Collection, Pasteur Institute of Iran, Karaj, Iran and were used in the protection experiments.
3.3. Experimental Animal
Female BALB/c mice, 6 - 8 weeks old (were obtained from the Pasteur institute of Iran, The Animal Center, Karaj, Iran) were acclimated and randomly distributed into study groups. The mice were kept in conventional animal facilities and received adequate water and food.
3.4. In Silico Design and Prediction of Recombinant Proteins
In silico prediction and design was performed based on conserved areas among different Brucella spp., (28); briefly, 3 antigenic determinants of Bp26 (25 amino acids 87 - 111), Omp31 (27 amino acids 48 - 74), and TF (485 amino acids, whole sequence) were selected and fused together by linkers (EAAAK). We fused these determinants to have a suitable immunogenic structure (a large protein) based on increasing the size of antigen to best stimulation of immune responses.
The alignment was performed using ClustalW software. Gene optimization to expression in Escherichia coli was performed by Codon Optimization online service; DNA/RNA GC Content Calculator was used for G/C% calculation, before and after optimization. Antigenicity was predicted by IEDB Analysis Resource and Vaccine Design Server. Physicochemical parameters were computed using Expasy’s Protparam, Protein Calculator v3.4, and Recombinant Protein Solubility Prediction. Protein solubility was predicted by DSSP and VADAR. T-cell epitopes prediction parameters; binding both MHC class I and II were analyzed by GPS-MBA Prediction System version 1.0 and immune epitope database. Conformational B-cell epitope was predicted with web server CBTOPE and The PSIPRED Protein sequence analysis workbench. Presence of possible allergenic sites was predicted using AlgPred and SDAP-Structural Database of Allergenic Proteins.
Analysis of the secondary structure of messenger RNA was predicted using the 'mfold' Web Server and RNA structure Web Servers for RNA Secondary Structure Prediction. Secondary structure, sequence analysis, and functional parameters of protein were computed with GOR IV secondary structure prediction method and Predict Protein server. Tertiary structure-3D and stability prediction of protein were performed by Deep View-Swiss-PdbViewer. Recombinant protein modeling was performed using I-TASSER server. Tertiary structure and 3D structures were validated by using ProSA-web. The stereo chemical quality of protein structure was validated by Ramachandran plot (Z-score) in PROCHECK (12, 28, 29).
3.5. Expression and Purification of Recombinant Protein
The recombinant gene was synthesized and subsequently cloned (Biomatik, Canada) into pET-28a (+) to construct pET-recombinant protein (pET-rTF/Bp26/Omp31). The pET-rTF/Bp26/Omp31 was transformed into E. coli BL21 (DE3) strain (Novagen-Merck KGaA, Germany). The transformed clones were inoculated into Luria Bertani (LB) medium (Merck KGaA, Germany), containing 50 μg/mL kanamycin (Sigma-Aldrich, Taufkirchen, Germany). The incubation was continued by agitation to 0.5 O.D. values at 600 nm; Isopropyl-β-D-1-Thiogalactopyranoside (IPTG) (Sigma-Aldrich, Germany) was added to induce the gene expression (optimum condition: IPTG concentration 700 μg/mL, 4 hours). The culture was harvested and suspended in lyses buffer (8 M urea, 0.1 M NaH2PO4, and 0.01 M Tris, pH = 8.0) containing protease inhibitors (Sigma-Aldrich, Germany). Recombinant protein was purified using Nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen, UK).
Proteins were eluted in 1ml buffer containing 200 mM imidazole and 500 μL MES buffer (20 mM) (Sigma-Aldrich, Germany). The purified protein (70 kDa) was monitored by SDS-PAGE (Bio-Rad, CA, USA) and its concentration was estimated by spectrophotometer (Biochrom, UK) and Bradford protein method measurement. Molecular weight (MW) of protein was determined by pre-stained protein ladder marker (SM0671, with 10 bands approx. 10, 15, 25, 35, 40, 55, 70, 100, 130, 170 kDa) (Thermo Fisher Scientific, USA). SDS-PAGE protein bands were transferred into nitrocellulose membrane (Sigma-Aldrich, Germany) and were then incubated with anti His-Tag antibody (Sigma-Aldrich, Germany). Membrane was then incubated with anti-mouse IgG-peroxidase (Sigma-Aldrich, Germany). The membrane was developed in diaminobenzidine (DAB) solution (Sigma-Aldrich, Germany). Upon visualizing the protein band, the reaction was stopped by adding distilled water (12, 28, 29).
3.6. Circular Dichroism (CD) Spectroscopy
To evaluate and determine the secondary structures of predicted (in silico) recombinant protein rTF/Bp26/Omp31 (0.25 mg/mL in PBS) circular dichroism (CD) was recorded on a JASCO J-810 spectrometer (Jasco, Inc., USA).
3.7. Immunization of Mice
Female BALB/c mice in each study group (n = 15) were immunized by the subcutaneous (s.c.) route of 20, 30 and 40 µg of rTF/Bp26/Omp31 (groups R-20, R-30 and R-40, respectively). On day 0, Complete Freund’s Adjuvants (CFA) and on day 14 and 28, Incomplete Freund’s Adjuvants (IFA) (Sigma-Aldrich, Germany) were mixed in equal amounts with rTF/Bp26/Omp31, respectively. Phosphate-buffered saline (PBS) mixed with IFA was administrated to negative control groups intraperitoneally (i.p.). Two other positive control groups were immunized on day 0 with 2 × 108 CFU B. abortus RB51 and 2 × 108 CFU B. melitensis Rev1. Sera were obtained 14, 28, and 45 days after the first immunization (12, 28, 29).
3.8. ELISA
Serum reactivity was determined by specific indirect enzyme-linked immunosorbent assay (ELISA) before and after the challenge of mice to measure immune serum anti-rTF/Bp26/Omp31 IgG, IgG1, and IgG2a levels. The high binding 96 Well Single-Break Strip ELISA Plates (Nunc-Immuno, Sigma-Aldrich, Germany) were coated with 10 μg purified recombinant protein in 100 μL PBS (pH 7.4) and then well blocked with 100 μL of 1% (m/v) bovine serum albumin. After washing with PBS-Tween, the plates were incubated with mice sera at 37°C for 1 hour. 100 μL/well of Horseradish peroxidase conjugated goat anti-mouse IgG, IgG1, or IgG2a antibodies (diluted 1:5000) was used for detection. After washing the wells and following the addition of 100 μL of o-phenylenediamine dichloride (Sigma-Aldrich, Germany) in phosphatecitrate buffer (pH 5.5) and H2O2 as a substrate, the plates were again incubated at 37°C for 15 minutes, in a dark place. Finally, the absorbance of wells was measured at O.D. (absorbance) 450 nm (BioTek, USA) after the development of color was stopped by the addition of 1N, H2SO4 (Merck KGaA, Germany). The results of immune mice were compared with the titer of antibody in serum controls (12, 28, 29).
3.9. Cytokine Responses
Thirty days after the ultimate immunization, under aseptic criteria, spleens of the study groups were removed and homogenized (n = 5/group). Splenocytes were suspended in RPMI 1640 medium (Thermo Fisher Scientific, MA, USA) with 2 mM L-glutamine, 10 mM HEPES (Sigma-Aldrich, Germany), 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, MA, USA), 1 mM 2-Mercoptoethanol (Sigma-Aldrich, Germany), and 1% of antibiotic-antimicotic solution (Invitrogen, New Zealand), supplementary. The cultures were incubated on a 96-well flat-bottom plate (Nunc-Immuno, Sigma-Aldrich, Germany), kept at 37°C, and with 5% CO2, for 72 hours. The splenocytes were cultured at 4 × 106 cell per well with 10 µg/mL purified rTF/Bp26/Omp31, 5 µg Concanavalin A (ConA) (Sigma-Aldrich, Germany), positive control, or with culture medium alone, as negative control. Interleukin 10 (IL-10), IL-4, Gamma interferon (IFN-γ) and (IL-12) were measured by ELISA using mouse ELISA kits according to manufacturer’s instruction (Bio-Techne, USA) (23, 24, 30).
3.10. Assessment of Protective Efficacy and Immunogenicity
On day 30, after the final injection, mice of each experimental groups (n = 5/group) were intraperitoneally challenged by virulent strains of 2 × 105 CFU/mL B. melitensis 16 M and B. abortus 544. One month after bacterial challenge, the infected mice were sacrificed and, aseptically, their spleens were separated. After homogenization of splenocytes in 1 mL of PBS, samples were plated in Brucella agar (Thermo Fisher Scientific, MA, USA) for 72 hours, at 37°C with 10% CO2 in a humidified atmosphere. The CFU count per spleen was determined and the results were presented as the mean logarithm (log) CFU ± error of the mean (SEM) per group. Log units of protection were obtained by subtracting the mean log CFU of the vaccinated group from the mean log CFU of the control group (23, 24, 30).
3.11. Statistical Analysis
The CFU results, CFU differences between experimental groups, and logarithm transformation were evaluated by ANOVA and Tukey’s post hoc test. Cytokines production and antibody titers were compared using the Mann-Whitney U-test (IBM SPSS Statistics 20 and Microsoft-Excel for graphs). Statistical significance was assumed at the P value < 0.05 level.
4. Results
4.1. Recombinant Protein Prediction and Production
Blast and alignment Sequence comparison, illustrated the highly conserved sequences among Brucella spp. Final construction of chimera: 1 - 485 (TF) amino acids (aa), 486 - 495 (EAAAKEAAAK-Linker) aa, 496 - 520 (Bp26) aa, 521 - 525 (EAAAK-Linker) aa, and 526 - 552 (Omp31) aa was performed by fusing the C terminal of TF, middle portion of Bp26 87-111, and N terminal of Omp31 48 - 74 in silico and synthesized in vitro (final designed structure of protein according to the suitable in silico conformational structure). It is important to know that due to in silico structure designing, we cannot generalize the results of 3 determinants alone to final designed structure, and thus the immunogenicity of these structures is not similar to original structure.
Gene optimization to best expression in E. coli was improved by changing the GC count from 51% in native form to 55% in reforming nucleotide. Prediction of antigenicity and linear epitope of antigen showed the antigenic determinant in several different sequences. MHC I and II classes binding sites, were determined in protein structure, in several positions. There was no presence of possible allergenic sites, based on the similarity of known epitopes. Physicochemical parameters of protein was computed approximately: molecular weight: ~ 65 kDa, theoretical pI: ~ 5.0, estimated half-life: > 10 hours (E. coli, in vivo), instability index: ~ 45.00 (regarding that chimeric protein was stable), aliphatic index: ~ 80.00 (a positive factor for the increase of thermo-stability) and ~ 100% chance of solubility when over expressed in E. coli.
There was no disorder in mRNA conformational structure, normal folding, and hairpin or pseudo knot in first nucleotides. Optimal secondary structure with a minimum free energy ~ -480 kcal/mol prepared a suitable ΔG in nucleotides. Protein secondary structure analysis showed that 58.70%, 7.07%, and 34.24% of sequences were alpha-helix, extended strand, and random coil, respectively. Tertiary structure of the protein showed a construction with 3 determined domains. Comparison of protein with native domain structures illustrated that the protein had acceptable stability (~ -14,000 Kcal/mol). This data was confirmed by the Ramachandran plot.
SDS-PAGE and Western blotting (using anti-His-tag antibodies) were used to analyze the expression of recombinant protein (Figure 1). Protein electrophoresis in Figure 1A shows the samples from the induced and un-induced cell lysates of E. coli BL21 (DE3). Western blotting analysis of purified recombinant protein showed the single band, corresponding to the expected size of rTF/Bp26/Omp31, 70 kDa (Figure 1).
Recombinant Protein Expression and Western Blotting
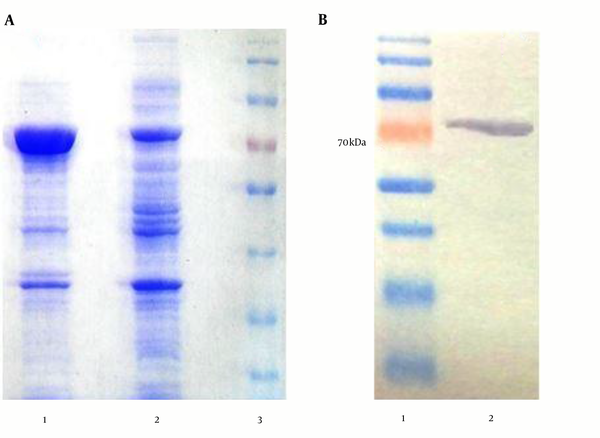
4.2. Antibody Response to the Recombinant Protein rTF/Bp26/Omp31 in Immunized BALB/c Mice
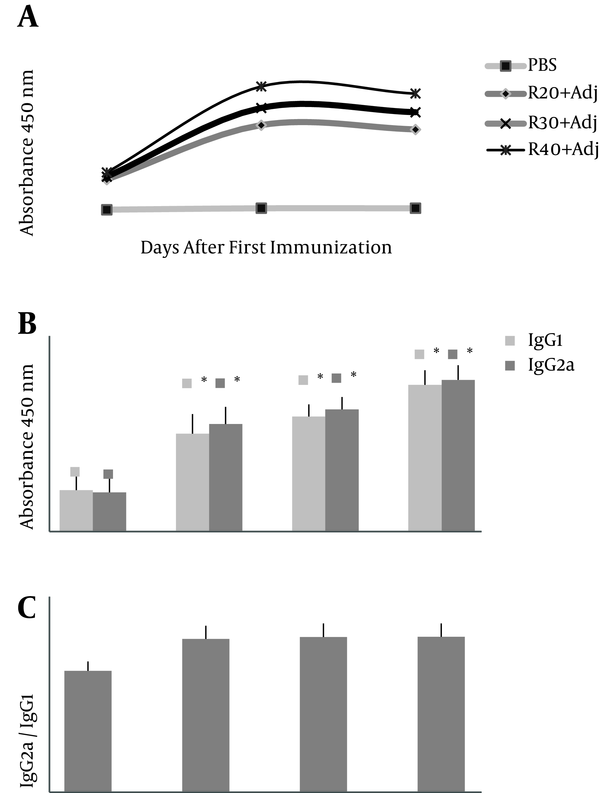
Antibody titers in ELISAs demonstrated (Figure 2A) that first administration of rTF/Bp26/Omp31 did not significantly (P > 0.05) increase the titers of total IgG antibodies versus negative control group-PBS (day 14). In contrast, a significant increase in the total IgG antibodies (P < 0.05) was observed in the positive control group after 2nd and 3rd administration (days 28 and 45) compared to the experimental groups (Figure 2A). Total IgG titers increased steadily and reached the highest amount at days 28 in groups R-40, R-30, and R-20, respectively (Figure 2A). Analysis of IgG isotype (IgG1 and IgG2a) indicated that the anti-rTF/Bp26/Omp31 detected in immunized mice was still predominant at day 45 after first injection and significantly higher in the immunized groups compared to the mice injected with PBS (P < 0.05) (Figure 2B). The specific IgG2a titers were higher than the specific IgG1 titers that indicate a Th1 response (IgG2a/IgG1 ratio 1.1, 1.06, and 1.03 in R-40, R-30, and R-20 groups, respectively) (Figure 2C).
Antibody Response to the Recombinant Protein rTF/Bp26/Omp31 in Immunized BALB/c Mice
4.3. Cytokines Production and Immune Responses
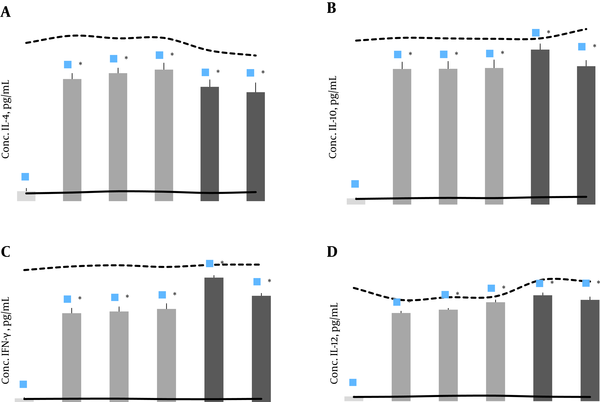
After in vitro stimulation with rTF/Bp26/Omp31 antigen, the cytokines production in the samples from the immunized animals was significantly higher than the negative experimental group (P < 0.05), and did not differ significantly (P > 0.05) with other positive control group vaccinated with B. abortus RB51 and B. melitensis Rev1 (Figure 3). Supernatants of splenocyte culture from immunized mice contained significantly high levels of IL-4, IL-10, IL-12 and IFN-γ (P < 0.05) in comparison with the negative control groups (Figure 3). The results indicate that vaccination with rTF/Bp26/Omp31 induced both humoral and cell-mediated immune responses by mixed T helper1 and 2 immune response, as demonstrated in production of all measured cytokines.
Cytokines Production
4.4. Induced Protection in BALB/c Mice Against B. melitensis and B. abortus Challenge
Significant decrease in the bacteria count in the culture of homogenized splenocytes was regarded as efficacy protection in immunized and control groups. Protection efficacy was calculated as the log10 of protection in spleen according to the difference of negative (PBS) group and immunized experimental groups. The recombinant protein showed significant protection at 30 days after challenging with 1.41, 1.49, and 1.61 log units of protection against B. melitensis 16M and 1.53, 1.55, and 1.63 log units of protection against B. abortus 544; these results were compatible to the live attenuated B. melitensis Rev.1 (2.1 log unit of protection) and B. abortus RB51 (1.96 log unit of protection) control vaccines results (Table 1). The results indicate the ability of rTF/Bp26/Omp31 to protect against infection when injected subcutaneously and the vaccine potential of immunization (P < 0.05).
Protection Against B. melitensis 16M and B. abortus 544 Challenge in Immunized Mice by rTF/Bp26/Omp31a,b
| Experimental Groups | log10 CFU of B. abortus 544 in Spleen | Protection Unit in Spleen (Log Units) | log10 CFU of B. melitensis 16M in Spleen | Protection Unit in Spleen (Log Units) |
|---|---|---|---|---|
| Negative control-PBS | 5.88 ± 0.55 | - | 5.23 ± 0.51 | - |
| R-20 + Adj | 4.35 ± 0.47 | 1.53c | 3.82 ± 0.33 | 1.41c |
| R-30 + Adj | 4.33 ± 0.33 | 1.55c | 3.74 ± 0.41 | 1.49c |
| R-40 + Adj | 4.25 ± 0.25 | 1.63c | 3.62 ± 0.31 | 1.61c |
| Rev.1 | - | - | 3.22 ± 0.19 | 2.01c |
| RB51 | 3.92 ± 0.21 | 1.96c | - | - |
5. Discussion
The development of subunit vaccines to protect against brucellosis is crucial to avoid the disadvantages of the used live attenuated vaccines B. melitensis Rev.1 as well as B. abortus RB51 and S19 (5, 10). New vaccines will be designed according to immune responses during a natural infection in animal models and identification of intracellular and cell surface immunodominant component of Brucella spp. (6, 9, 11, 31). Previous studies showed that multivalent recombinant vaccines can elicit a vigorous stimulation in immune response and better protection efficacy compared with the pertinent univalent vaccines (8, 9, 14). Omp31 have a highly conserved hydrophilic loop (48 - 74 residues) regarded as Th1 protective epitope (30). Furthermore, antigenic determinants of TF and Bp26 (87-111 residues) can induce a T-cell mediated response (17-20, 27, 32). TF, Bp26, and Omp31 immunogenicity potential is considered as a good component for a subunit vaccine design.
Immunity against Brucella is mediated by mixed T helper1 and 2 immune response and both B and T cell-mediated immunity (HMI and CMI) are derived (13, 15, 33). Derivative cytokines of Th1 and 2 cells including IFN-γ, IL-12, as well as IL-10 and IL-4 modulate the immune response against intracellular Brucella (13, 15, 34). IFN-γ has a crucial role in the control of intracellular bacteria via macrophages activation and protective IgG2a antibody responses (19, 34). After binding of Fc portion of IgG2a to surface Fc-receptors on the phagocytes, antimicrobial responses (e.g., phagocytosis, synthesis of cytokines, and inflammatory mediators release) are performed (13, 15). Specific response of IgG1 antibodies is related to IL-4 and IL-10 synthesis, that regulate the HMI responses and is critical to the prevention of this infection in the initial stage of brucellosis (13, 33).
In this study we evaluated the induced protective efficacy and immune response by a recombinant chimera protein rTF/Bp26/Omp31 in BALB/c mice. Evaluation of humoral and cellular immune responses to Bp26 and Omp31 epitopes in the attenuated Brucella melitensis vaccine showed the efficacy of this component in immunity against Brucella (19). In another study, the protective effect and immune responses against Omp31 and Bp26 was evaluated in mice challenged with Brucella (21). It has been shown that Brucella melitensis Rev.1 vaccine single and double deletion mutants of the Bp26 and Omp31 affect the protective efficacy against brucellosis (32). However in many previous studies, the role of TF, Bp26, and Omp31 immunodominants in immune responses was shown (16, 17, 19, 20, 22, 25, 27, 30).
To evaluate the stimulation type of immunity, production and synthesis of IgG isotypes, IFN-γ, IL-4, IL-10, and IL-12 were done by ELISA. Results showed the ability of rTF/Bp26/Omp31 to induce an intense IgG response in comparison with control groups (P < 0.05). Since the isotype of IgG response is related to the kind of cytokines production by Th1 and 2 cells, we determined the IgG1 and IgG2a titers in response to rTF/Bp26/Omp31 injection in mice. Significant (P < 0.05) increased titers of IgG1 and IgG2a were detectable in the sera of the immunized mice in comparison to the negative group (PBS) with shifted responses to IgG2a production; the IgG2a/IgG1 ratio was, approximately, equal to 1 in immunized mice with recombinant protein (higher titers of IgG2a). Since the profile of antibody isotype response is a reaction to the T helper cell types, these results indicate the induced Th1 responses against rTF/Bp26/Omp31 vaccination.
Cassataro et al. showed that vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection (30). It is important to know that Th1 responses and IgG2a productions are essential for eradication of Brucella spp. infections, by the cell mediated immunity, cytokine production, and facility of phagocytes (15, 33, 34). To evaluate the cytokines responses to rTF/Bp26/Omp31, production levels of IL-4, IL-10, IL-12, and IFN-γ were measured after splenocytes stimulation. Increased levels of IL-12, IFN-γ, IL-4, and IL-10 suggested a combined Th1 and 2 immune response (Th1 by IL-12 and IFN-γ and Th2 by IL-4 and IL-10). Results of cytokines production in the immunized group with rTF/Bp26/Omp31 in comparison with RB51, Rev.1, and PBS-negative group showed the significant increased level of these cytokines production in the study group (P < 0.05).
Additionally, protection units in the spleen (log units) showed significant induced protection against rTF/Bp26/Omp31 in experimental mice (Table 1) (log units of protection were obtained by subtracting the mean log CFU of the vaccinated group from the mean log CFU of the control group). Immunized mice with B. abortus 544 (1.96 log units) in comparison to mice immunized by 20, 30, and 40 µg rTF/Bp26/Omp31, showed the 1.53, 1.55, and 1.63 log units of protection against B. abortus 544, respectively; furthermore, 1.41, 1.49, and 1.61 log units in mice immunized by 20, 30 and 40 µg rTF/Bp26/Omp31, respectively, compared with B. melitensis 16M (2.01 log units) showed the protection efficacy. However protection units showed the significant increased level of protection (P < 0.05) in study group (Table 1).
6. Conclusion
Our results suggest that this recombinant protein could be used as a potential subunit vaccine candidate against Brucella spp. with remarkable ability in inducing both types of humoral and cellular-mediated immunity compared with common live attenuated B. abortus RB51 and B. melitensis Rev.1 vaccines.
Acknowledgements
References
-
1.
Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140(3-4):392-8. [PubMed ID: 19604656]. https://doi.org/10.1016/j.vetmic.2009.06.021.
-
2.
Fugier E, Pappas G, Gorvel JP. Virulence factors in brucellosis: implications for aetiopathogenesis and treatment. Expert Rev Mol Med. 2007;9(35):1-10. [PubMed ID: 18088444]. https://doi.org/10.1017/S1462399407000543.
-
3.
Pappas G. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents. 2010;36 Suppl 1:S8-11. [PubMed ID: 20696557]. https://doi.org/10.1016/j.ijantimicag.2010.06.013.
-
4.
Nicoletti P. Brucellosis: past, present and future. Prilozi. 2010;31(1):21-32. [PubMed ID: 20703181].
-
5.
Avila-Calderon ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodriguez A. A history of the development of Brucella vaccines. Biomed Res Int. 2013;2013:743509. [PubMed ID: 23862154]. https://doi.org/10.1155/2013/743509.
-
6.
Schurig GG, Sriranganathan N, Corbel MJ. Brucellosis vaccines: past, present and future. Vet Microbiol. 2002;90(1-4):479-96. [PubMed ID: 12414166].
-
7.
Martins Rda C, Irache JM, Gamazo C. Acellular vaccines for ovine brucellosis: a safer alternative against a worldwide disease. Expert Rev Vaccines. 2012;11(1):87-95. [PubMed ID: 22149711]. https://doi.org/10.1586/erv.11.172.
-
8.
Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. 2010;34(3):379-94. [PubMed ID: 20180858]. https://doi.org/10.1111/j.1574-6976.2010.00211.x.
-
9.
Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. Progress in Brucella vaccine development. Front Biol (Beijing). 2013;8(1):60-77. [PubMed ID: 23730309]. https://doi.org/10.1007/s11515-012-1196-0.
-
10.
Olsen SC. Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech. 2013;32(1):207-17. [PubMed ID: 23837378].
-
11.
Zhao Z, Yan F, Ji W, Luo D, Liu X, Xing L, et al. Identification of immunoreactive proteins of Brucella melitensis by immunoproteomics. Sci China Life Sci. 2011;54(9):880-7. [PubMed ID: 21922434]. https://doi.org/10.1007/s11427-011-4218-2.
-
12.
He Y, Xiang Z. Bioinformatics analysis of Brucella vaccines and vaccine targets using VIOLIN. Immunome Res. 2010;6 Suppl 1. S5. [PubMed ID: 20875156]. https://doi.org/10.1186/1745-7580-6-S1-S5.
-
13.
Costa Oliveira S, Costa Macedo G, Augusto de Almeida L, Souza de Oliveira F, Onate A, Cassataro J, et al. Recent Advances in Understanding Immunity Against Brucellosis: Application for Vaccine Development. Open Vet Sci J. 2010;4(1):102-8. https://doi.org/10.2174/1874318801004010102.
-
14.
Dorneles EM, Sriranganathan N, Lage AP. Recent advances in Brucella abortus vaccines. Vet Res. 2015;46:76. [PubMed ID: 26155935]. https://doi.org/10.1186/s13567-015-0199-7.
-
15.
He Y. Analyses of Brucella Pathogenesis, Host Immunity, and Vaccine Targets using Systems Biology and Bioinformatics. Front Cel Infect Microbiol. 2012;2. https://doi.org/10.3389/fcimb.2012.00002.
-
16.
Deuerling E, Patzelt H, Vorderwulbecke S, Rauch T, Kramer G, Schaffitzel E, et al. Trigger Factor and DnaK possess overlapping substrate pools and binding specificities. Mol Microbiol. 2003;47(5):1317-28. [PubMed ID: 12603737].
-
17.
Yang X, Walters N, Robison A, Trunkle T, Pascual DW. Nasal immunization with recombinant Brucella melitensis bp26 and trigger factor with cholera toxin reduces B. melitensis colonization. Vaccine. 2007;25(12):2261-8. [PubMed ID: 17239499]. https://doi.org/10.1016/j.vaccine.2006.12.004.
-
18.
Ghasemi A, Salari MH, Zarnani AH, Pourmand MR, Ahmadi H, Shirazi MH, et al. Immunogenicity assessment of Brucella mellitensis HSP and TF proteins by immunized rabbit serum. Iran J Allergy Asthma Immunol. 2013;12(2):192-4. [PubMed ID: 23754360].
-
19.
Wang W, Wu J, Qiao J, Weng Y, Zhang H, Liao Q, et al. Evaluation of humoral and cellular immune responses to BP26 and OMP31 epitopes in the attenuated Brucella melitensis vaccinated sheep. Vaccine. 2014;32(7):825-33. [PubMed ID: 24370708]. https://doi.org/10.1016/j.vaccine.2013.12.028.
-
20.
Grillo MJ, Marin CM, Barberan M, de Miguel MJ, Laroucau K, Jacques I, et al. Efficacy of bp26 and bp26/omp31 B. melitensis Rev.1 deletion mutants against Brucella ovis in rams. Vaccine. 2009;27(2):187-91. [PubMed ID: 19007836]. https://doi.org/10.1016/j.vaccine.2008.10.065.
-
21.
Gupta VK, Radhakrishnan G, Harms J, Splitter G. Invasive Escherichia coli vaccines expressing Brucella melitensis outer membrane proteins 31 or 16 or periplasmic protein BP26 confer protection in mice challenged with B. melitensis. Vaccine. 2012;30(27):4017-22. [PubMed ID: 22546330]. https://doi.org/10.1016/j.vaccine.2012.04.036.
-
22.
Estein SM, Fiorentino MA, Paolicchi FA, Clausse M, Manazza J, Cassataro J, et al. The polymeric antigen BLSOmp31 confers protection against Brucella ovis infection in rams. Vaccine. 2009;27(48):6704-11. [PubMed ID: 19748579]. https://doi.org/10.1016/j.vaccine.2009.08.097.
-
23.
Cassataro J, Pasquevich KA, Estein SM, Laplagne DA, Zwerdling A, de la Barrera S, et al. A DNA vaccine coding for the chimera BLSOmp31 induced a better degree of protection against B. ovis and a similar degree of protection against B. melitensis than Rev.1 vaccination. Vaccine. 2007;25(32):5958-67. [PubMed ID: 17600596]. https://doi.org/10.1016/j.vaccine.2007.05.049.
-
24.
Cassataro J, Pasquevich K, Bruno L, Wallach JC, Fossati CA, Baldi PC. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough Brucellae. Clin Diagn Lab Immunol. 2004;11(1):111-4. [PubMed ID: 14715555].
-
25.
Diaz AG, Clausse M, Paolicchi FA, Fiorentino MA, Ghersi G, Zylberman V, et al. Immune response and serum bactericidal activity against Brucella ovis elicited using a short immunization schedule with the polymeric antigen BLSOmp31 in rams. Vet Immunol Immunopathol. 2013;154(1-2):36-41. [PubMed ID: 23643287]. https://doi.org/10.1016/j.vetimm.2013.04.003.
-
26.
Cloeckaert A, Baucheron S, Vizcaino N, Zygmunt MS. Use of Recombinant BP26 Protein in Serological Diagnosis of Brucella melitensis Infection in Sheep. Clin Vaccine Immunol. 2001;8(4):772-5. https://doi.org/10.1128/cdli.8.4.772-775.2001.
-
27.
Qiu J, Wang W, Wu J, Zhang H, Wang Y, Qiao J, et al. Characterization of periplasmic protein BP26 epitopes of Brucella melitensis reacting with murine monoclonal and sheep antibodies. PLoS One. 2012;7(3). e34246. [PubMed ID: 22457830]. https://doi.org/10.1371/journal.pone.0034246.
-
28.
Ghasemi A, Ranjbar R, Amani J. In silico analysis of chimeric TF, Omp31 and BP26 fragments of Brucella melitensis for development of a multi subunit vaccine candidate. Iran J Basic Med Sci. 2014;17(3):172-80. [PubMed ID: 24847419].
-
29.
Sekhavati MH, Heravi RM, Tahmoorespur M, Yousefi S, Abbassi-Daloii T, Akbari R. Cloning, molecular analysis and epitopics prediction of a new chaperone GroEL Brucella melitensis antigen. Iran J Basic Med Sci. 2015;18(5):499-505. [PubMed ID: 26124937].
-
30.
Cassataro J, Estein SM, Pasquevich KA, Velikovsky CA, de la Barrera S, Bowden R, et al. Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infect Immun. 2005;73(12):8079-88. [PubMed ID: 16299302]. https://doi.org/10.1128/IAI.73.12.8079-8088.2005.
-
31.
Yang Y, Wang L, Yin J, Wang X, Cheng S, Lang X, et al. Immunoproteomic analysis of Brucella melitensis and identification of a new immunogenic candidate protein for the development of brucellosis subunit vaccine. Mol Immunol. 2011;49(1-2):175-84. [PubMed ID: 21943783]. https://doi.org/10.1016/j.molimm.2011.08.009.
-
32.
Cloeckaert A, Jacques I, Grillo MJ, Marin CM, Grayon M, Blasco JM, et al. Development and evaluation as vaccines in mice of Brucella melitensis Rev.1 single and double deletion mutants of the bp26 and omp31 genes coding for antigens of diagnostic significance in ovine brucellosis. Vaccine. 2004;22(21-22):2827-35. [PubMed ID: 15246618]. https://doi.org/10.1016/j.vaccine.2004.01.001.
-
33.
Baldwin CL, Goenka R. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit Rev Immunol. 2006;26(5):407-42. [PubMed ID: 17341186].
-
34.
Skendros P, Boura P. Immunity to brucellosis. Revue Scientifique et Technique de l'OIE. 2013;32(1):137-47. https://doi.org/10.20506/rst.32.1.2190.