Abstract
Background:
Salmonella is an important foodborne pathogen that causes diarrhea in humans worldwide.Objectives:
This study aimed to determine the serotype distribution, antibiotic-resistant genes, and Salmonella pathogenicity island (SPI) genes of clinical isolates of Salmonella in Weifang.Methods:
A total of 111 Salmonella strains were collected from Weifang People’s Hospital between 2018 and 2020 and subjected to serotyping using the Kauffmann-White antigen table. Meanwhile, the polymerase chain reaction detected eleven SPI1-6 genes and six antibiotic resistance genes.Results:
Among the 111 Salmonella strains, 17 serotypes were identified, with S. Typhimurium, S. Typhi, and S. Enteritidis being the most prevalent. The hilA, ssaB, sseC, marT, siiE, pipB, sopB, and pagN SPI1-6 genes were all found during analysis. The InvA, misL, and siiD genes were detected at 98.2, 97.30, and 97.30% rates, respectively. Also, sul2 and blaTEM were the most prevalent antibiotic resistance genes in this investigation, accounting for 68.47 and 21.62% of the total, respectively.Conclusions:
Salmonella isolated from the clinical samples was found to have a diversity of serotypes and possessed various SPI and antibiotic resistance genes.Keywords
Salmonella Serotype Salmonella Pathogenicity Island (SPI) Antibiotic Resistance Gene
1. Background
Salmonella is a dominant genus of Enterobacteriaceae and a foodborne pathogen that can cause food poisoning (1). Salmonella is widely distributed in the environment in variable serotypes. There are more than 20 serotypes known to cause zoonosis. About 70 to 80% of the reported food poisoning cases in China are caused by Salmonella (2). Currently, three generations of cephalosporins and quinolones are mainly used in the clinical treatment of Salmonella infection. With the increase in use time and frequency, many drug-resistant Salmonella isolates have appeared (3). Sulfonamides were the first drugs to be used in veterinary medicine in therapeutic doses (4). Sulfonamides were a high priority of veterinary medicines due to their high potential to reach the environment (5). High sulfonamide concentrations increase the risk of food chain contamination (6).
Currently, the drug resistance mode of Salmonella is to several antibiotics at the same time, and multidrug-resistant bacteria exist widely globally (7). Therefore, β-lactam resistance genes (blaTEM, blaSHV, and blaOXA2), the sulfonamide resistance gene (sul2), and fluoroquinolones resistance genes (qnrA and qnrB) were selected in this experiment. Studying bacterial antibiotic resistance at the molecular level can help expose the antibiotic resistance mechanism of bacteria. Bacterial pathogenicity is governed by virulence factors. The pathogenicity island aims to investigate these gene clusters, closely associated with bacterial pathogenicity and virulence factors (8). The Salmonella pathogenicity island (SPI) encodes virulence factors found throughout the Salmonella genome, where SPI1-6 is a pathogenic island in Salmonella. However, distribution of SPI1-6 genes varies between serotypes (9), although SPI1-6 is an essential pathogenicity island (10). Consequently, the analysis of the SPI gene may prove helpful in comprehending the pathogenicity and virulence factors of bacteria.
2. Objectives
Analyzing and studying the prevalence, serotype, antibiotic resistance gene, and SPI1-6 gene distribution of Salmonella in Weifang People’s Hospital can help understand the molecular epidemiological characteristics of Salmonella, guide clinical rational antibiotic use, and provide data support and a theoretical foundation for disease prevention and control.
3. Methods
3.1. Bacterial Isolates
The study was performed on a collection of clinically identified strains of Salmonella from Weifang People’s Hospital between 2018 and 2020. The duplicated strains of the same patient at the same site during hospitalization were excluded. The VITEK 2-Compact system (BioMerieux, France) was used to identify the bacteria in all strains.
3.2. Serotype Profiling
The strains were inoculated onto the plate and incubated overnight at 37°C. The O and H antigens of the strains were detected according to the instructions of the Salmonella serum diagnostic kit and compared with the Kauffmann-White antigen table to determine the serotype of Salmonella.
3.3. Genome DNA Extraction
All strains were streaked onto Luria Bertani agar plates (Oxford, UK) and incubated at 37°C overnight. A single colony was selected for DNA extraction, and total genomic DNA was obtained from Salmonella following the manufacturer’s instructions using the EZ-10 Spin Column Bacterial Genomic DNA Miniprep Kit (Bio Basic, Canada).
3.4. Determination of Antibiotic Resistance Genes and SPI1-6 Genes
Six pairs of antibiotic-resistant gene and eleven pairs of SPI1-6 virulence gene amplification primers were designed based on the gene sequences in GenBank and the literature (5, 11-18). All PCR products were sequenced using primer walking, starting with 3CS and 5CS primers (Table 1). The primers were designed by Sangon Biotechnology Co., Ltd. (Shanghai, China). PCR parameters were preset at 95°C for 5 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 90 s, and 72°C for 10 min. The amplified products were analyzed by electrophoresis on a 1% agarose gel, and the results were visualized using a gel imaging system.
Primer Sequences of SPI Gene and Antibiotic Resistance Gene of Salmonella
| Genes | Primer Sequence (5’-3’) | Length (bp) | |
|---|---|---|---|
| SPI-1 | hilA | F:GACAGAGCTGGACCACAATAAGACA | 312 |
| R:GAGCGTAATTCATCGCCTAAAC | |||
| InvA | F: GTG AAA TTA TCG CCA CGT TCG GGCAA | 284 | |
| R: TCATCGCACCGTCAAAGGAACC | |||
| SPI-2 | ssaB | F:GATATCAGGGCCGAAGGTAAT | 294 |
| R:GCAAGTTAAAGCCAGGTGTTT | |||
| sseC | F:ATGAATCGAATTCACAGTAA | 1445 | |
| R:TTAAGCGCGATAGCCAGCTA | |||
| SPI-3 | marT | F:CGTCGTCTCACAACAAACATTC | 556 |
| R:CTGACAAATCAATGCCGTAACC | |||
| misL | F:CACTGACCTGACGCTGAATAG | 946 | |
| R:GACTTGACCACGGGAATGATAG | |||
| SPI-4 | siiE | F:TTTTTTGCCGATCAAAATTCTGTA | 750 |
| R:TATACTATCATCTTTGCTACCGCT | |||
| siiD | F:GTCAGGGCGTTATCACTACTAAA | 826 | |
| R: TTCACATCGGCCAGCATAG | |||
| SPI-5 | pipB | F:CCTGTGGTGGAGTAAGAAGAAG | 599 |
| R:GTCAGTTAAGTCTGAGCCGAATA | |||
| sopB | F:TCACTAAAAACCCAGGAGGCTTTT | 1000 | |
| R:CGCCATCTTTATTGCGGATTTTTA | |||
| SPI-6 | pagN | F:TTCCAGCTTCCAGTACGTTTAG | 440 |
| R:GCCTTTGTGTCTGCATCATAAG | |||
| β-Lactams | blaTEM | F:TCCGCTCATGAGACAATAACC | 931 |
| R:TGGTCTGACAGTTACCAATGC | |||
| blaOXA2 | F:ATACACTTTTTGCACTTGATGCAG | 478 | |
| R:TGAAAAGATCATCCATTCTGTTTG | |||
| blaSHV | F:F-TGGTTATGCGTTATATTCGCC | 868 | |
| R:GGTTAGCGTTGCCAGTGCT | |||
| Sulphonamides | sul2 | F: GCAGGCGCGTAAGCTGA | 657 |
| R: GGCTCGTGTGTGCGGATG | |||
| Quinolones | qnrA | F:ATTTCTCACGCCAGGATTTG | 516 |
| R:GATCGGCAAAGGTTAGGTCA | |||
| qnrB | F:GTTGGCGAAAAAATTGACAGAA | 526 | |
| R:ACTCCGAATTGGTCAGATCG |
4. Results
4.1. Isolation and Serotype Profiling of Salmonella Strains
In this study, 111 strains of Salmonella were selected from Weifang People’s Hospital inpatients. A total of 17 serotypes were found. The serotypes were mainly S. Typhimurium, S. Typhi, and S. Enteritidis. The serotype profiles are provided in Table 2.
Serotype Profiling of Salmonella
| Salmonella Serotypes | Isolates/Proportions, No. (%) |
|---|---|
| S. Typhimurium | 32 (28.83) |
| S. Typhi | 24 (21.62) |
| S. Enteritidis | 20 (18.02) |
| S. Paratyphi B | 9 (8.12) |
| S. Paratyphi C | 6 (5.41) |
| S. Paratyphi A | 2 (1.80) |
| S. Dublin | 3 (2.70) |
| S. Liverpool | 3 (2.70) |
| S. Javiana | 2 (1.80) |
| S. Derby | 3 (2.70) |
| S. Gallinarum | 1 (0.90) |
| S. Concord | 1 (0.90) |
| S. Aberdeen | 1 (0.90) |
| S. Choleraesuis | 1 (0.90) |
| S. London | 1 (0.90) |
| S. Bovismorbificans | 1 (0.90) |
| S. Weltevreden | 1 (0.90) |
4.2. Molecular Detection of Salmonella Antibiotic Resistance Genes
Three antibiotic classes and six antibiotic resistance genes were amplified from the 111 Salmonella strains. The carrying rates of β-lactam resistance genes blaTEM, blaSHV, and blaOXA2 were 21.62%, 0.9%, and 0.9%, respectively. The carrying rate of the sulfonamide resistance gene sul2 was estimated to be 68.47%. In comparison, the qnrA band rate of the resistance gene in quinolones-resistant strains was 4.50%, without detection of qnrB (Table 3).
Antibiotic Resistance Gene Prevalence in Salmonella
| Antimicrobial Agents, Genes | Gene Prevalence, No. (%Rate) |
|---|---|
| β-Lactams | |
| blaTEM | 24 (21.62) |
| blaOXA2 | 1 (0.9) |
| blaSHV | 1 (0.9) |
| Sulphonamides | |
| sul2 | 76 (68.47) |
| Quinolones | |
| qnrA | 5 (4.50) |
| qnrB | 0 (0) |
The antibiotic resistance genes varied among various serotypes (Figure 1). Among them, only one strain, i.e., S. Enteritidis, was found to carry blaSHV and blaOXA2. The blaTEM gene was mainly present in S. Typhimurium, S. Typhi, and S. Enteritidis. Different serotypes of Salmonella had higher carrying rates of the sul2 gene, mainly found in S. Typhimurium, S. Typhi, S. Enteritidis, and S. Paratyphi B. Four strains of S. Enteritidis and one strain of S. Typhimurium carried qnrA (Table 4).
The distribution of the antibiotic resistance genes and the SPI1-6 genes in Salmonella
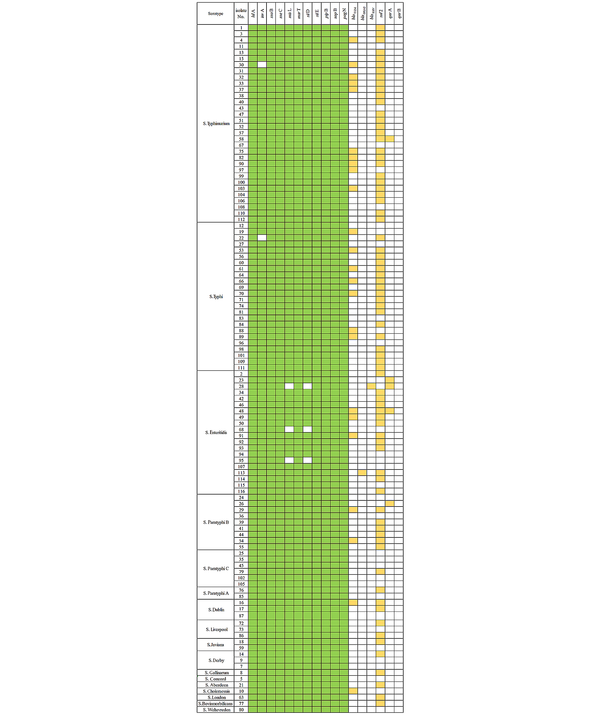
| Salmonella Serotypes | blaTEM | blaOXA2 | blaSHV | sul2 | qnrA | qnrB |
|---|---|---|---|---|---|---|
| S. Typhimurium | 10 (31.25) | 0 (0) | 0 (0) | 27 (84.38) | 1 (3.13) | 0 (0) |
| S. Typhi | 7 (29.17) | 0 (0) | 0 (0) | 18 (75.00) | 0 (0) | 0 (0) |
| S. Enteritidis | 3 (15.00) | 1 (5) | 1 (5) | 13 (65.00) | 4 (20) | 0 (0) |
| S. Paratyphi B | 2 (22.22) | 0 (0) | 0 (0) | 6 (66.67) | 0 (0) | 0 (0) |
| S. Paratyphi C | 0 (0) | 0 (0) | 0 (0) | 1 (16.67) | 0 (0) | 0 (0) |
| S. Paratyphi A | 0 (0) | 0 (0) | 0 (0) | 1 (50.00) | 0 (0) | 0 (0) |
| S. Dublin | 1 (33.33) | 0 (0) | 0 (0) | 2 (66.67) | 0 (0) | 0 (0) |
| S. Liverpool | 0 (0) | 0 (0) | 0 (0) | 2 (66.67) | 0 (0) | 0 (0) |
| S. Javiana | 0 (0) | 0 (0) | 0 (0) | 1 (50.00) | 0 (0) | 0 (0) |
| S. Derby | 0 (0) | 0 (0) | 0 (0) | 1 (33.33) | 0 (0) | 0 (0) |
| S. Gallinarum | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| S. Concord | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| S. Aberdeen | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| S. Choleraesuis | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| S. London | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| S. Bovismorbificans | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| S. Weltevreden | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
4.3. Molecular Detection of Salmonella Pathogenicity Island Genes
In the 111 Salmonella strains, 11 SPI1-6 genes were detected. SPI genes hilA, ssaB, sseC, marT, siiE, pipB, sopB, and pagN were detected in all Salmonella strains. The carrying rates of virulence genes invA, misL, and siiD were estimated to be 98.2%, 97.30%, and 97.30%, respectively (Table 5). The carrying rates of Salmonella virulence genes varied among various serotypes (Figure 1). The carrying rate of invA in S. Typhimurium and S. Typhi was found to be 96.88% and 95.83%, respectively, while the carrying rate of misL and siiD in S. Enteritidis was found to be 85% each. All other virulence genes had a carrying rate of 100% (Table 6).
SPI1-6 Gene Prevalence in Salmonella
| Pathogenicity Islands, Genes | Gene Prevalence, No. (%Rate) |
|---|---|
| SPI-1 | |
| hilA | 111 (100) |
| invA | 109 (98.20) |
| SPI-2 | |
| ssaB | 111 (100) |
| sseC | 111 (100) |
| SPI-3 | |
| misL | 108 (97.30) |
| marT | 111 (100) |
| SPI-4 | |
| siiD | 108 (97.30) |
| siiE | 111 (100) |
| SPI-5 | |
| pipB | 111 (100) |
| sopB | 111 (100) |
| SPI-6 | |
| pagN | 111 (100) |
| Salmonella Serotypes | hilA | invA | ssaB | sseC | misL | marT | siiD | siiE | pipB | sopB | pagN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. Typhimurium | 32 (100) | 31 (96.88) | 31 (100) | 32 (100) | 32 (100) | 32 (100) | 32 (100) | 32 (100) | 32 (100) | 32 (100) | 32 (100) |
| S. Typhi | 24 (100) | 23 (95.83) | 24 (100) | 24 (100) | 24 (100) | 24 (100) | 24 (100) | 24 (100) | 24 (100) | 24 (100) | 24 (100) |
| S. Enteritidis | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 17 (85) | 20 (100) | 17 (85) | 20 (100) | 20 (100) | 20 (100) | 20 (100) |
| S. Paratyphi B | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) | 9 (100) |
| S. Paratyphi C | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| S. Paratyphi A | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| S. Dublin | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| S. Liverpool | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| S. Javiana | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| S. Derby | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| S. Gallinarum | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| S. Concord | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| S. Aberdeen | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| S. Choleraesuis | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| S. London | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| S. Bovismorbificans | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| S. Weltevreden | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
5. Discussion
There are currently around 2600 known Salmonella serotypes with a complex nature. According to the World Health Organization’s global Salmonella monitoring system, S. Enteritidis and S. Typhimurium are the most prevalent bacteria responsible for foodborne disease (19, 20). This study showed that the 111 strains of Salmonella presented 17 serotypes, indicating that the serotypes in Chinese hospitals are diverse and abundant, with a major share of S. Typhimurium, S. Typhi, and S. Enteritidis, accounting for 68.47% of them (76/111). Also, S. Typhimurium and S. Enteritidis accounted for 28.83 and 18.02% of the serotypes, respectively, close to the rates reported in a previous study by Xu et al. (21). However, S. Typhi was undetected in Xu et al.’s study, but in this study, S. Typhi accounted for 21.62% of the serotypes. Compared to Salmonella serotypes in other regions of China, the proportion of S. Typhi in Weifang People’s Hospital is higher than the hospitals in other regions (21, 22). It can be concluded that serotypes are different in different regions of China and among patients with severe Salmonella infection.
Virulence is the root cause of Salmonella infection, elicited by the interaction of several virulence genes. SPI mainly encodes Salmonella virulence genes on the chromosome (23). More than 20 types of SPI have been discovered so far. SPI1 is required for Salmonella to invade host non-phagocytes (24), and SPI2 primarily regulates Salmonella multiplication in phagocytes and epithelial cells. SPI3 and SPI4 aid Salmonella survival and adherence to the surface of polarized cells, while SPI5 encodes an effector protein released via the type III secretion system encoded by SPI1 and SPI2 (25). Also, SPI6 encodes type VI secret system-related proteins (26). These virulence factors aid Salmonella invasion, reproduction, virulence, and transmission in a complex environment (27).
This study selected virulence genes from SPI1 to SPI6 as target genes for PCR amplification. The carrying rates of hilA, ssaB, sseC, marT, siiE, pipB, sopB, and pagN were 100%, and the carrying rates of invA, misL, and siiD were found to be 98.2%, 97.30%, and 97.30%, respectively. This study showed that the carrying rate of SPI1-6 genes was relatively high. Fabrega and Vila discovered that several virulence genes were involved in the expression. The higher virulence gene carrying rate translates into greater potential pathogenicity of Salmonella (28), implying that Salmonella harboring in these virulence genes is highly pathogenic.
In this experiment, five of the six drug resistance genes were detected, among which sul2 and blaTEM had the highest detection rates. The sul genes are found in plasmids and are associated with ubiquitous and long-known sulfonamide resistance Gram-negative bacteria (29). The detection results of the antibiotic resistance genes of the 111 strains of Salmonella revealed that the detection rate of the sulfonamide resistance gene sul2 was 68.47%, which was similar to the rate reported by Adesiji et al. (30). Due to the high detection rate of sul2, sulfonamides should be used with caution to prevent the spread of antibiotic resistance and the formation of multidrug-resistant strains. A very important factor of Salmonellaβ-lactam drug resistance is β-lactamase production. Bacteria producing β-lactamase can make hydrolytic inactivation of β-lactam antibiotics, and common types are blaTEM, blaOXA, and blaSHV. This experiment detected blaTEM, blaSHV, and blaOXA2 in Salmonella. The detection rate of the blaTEM gene was estimated to be 21.62%, close to the values reported by Shitta (31). One strain of S. Enteritidis was found to carry blaSHV and blaOXA2. Thus, these strains have the potential for β-lactam antibiotic resistance. According to the detection of drug resistance genes in this study, blaTEM has a high carrying rate of β-lactam antibiotic resistance genes, which should be taken into consideration.
In contrast, five strains of Salmonella were resistant to quinolones (the qnrA gene) with zero detection rate of qnrB, indicating that the resistance rate of Salmonella in Weifang People’s Hospital to quinolones was low, allowing to prioritize the treatment of Salmonella infection. Different serotypes of Salmonella have different carrying rates of antibiotic resistance genes. blaTEM and sul2 were the main drug resistance genes detected in S. Typhimurium, S. Typhi, and S. Paratyphi B. Appropriate antibiotics can be chosen based on distinct serotypes, allowing for more effective treatment of Salmonella infection.
5.1. Conclusions
The results revealed that Salmonella serotypes from Weifang People’s Hospital inpatients were widely spread. The detection rate of antibiotic-resistant genes and the carrying rate of SPI1-6 genes were high. Our findings necessitate strengthening the investigation of Salmonella molecular epidemiology and reducing the emergence of Salmonella antibiotic resistance.
References
-
1.
Foley SL, Zhao S, Walker RD. Comparison of molecular typing methods for the differentiation of Salmonella foodborne pathogens. Foodborne Pathog Dis. 2007;4(3):253-76. [PubMed ID: 17883310]. https://doi.org/10.1089/fpd.2007.0085.
-
2.
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882-9. [PubMed ID: 20158401]. https://doi.org/10.1086/650733.
-
3.
Crump JA, Medalla FM, Joyce KW, Krueger AL, Hoekstra RM, Whichard JM, et al. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother. 2011;55(3):1148-54. [PubMed ID: 21199924]. [PubMed Central ID: PMC3067073]. https://doi.org/10.1128/AAC.01333-10.
-
4.
Lees P, Pelligand L, Giraud E, Toutain PL. A history of antimicrobial drugs in animals: Evolution and revolution. J Vet Pharmacol Ther. 2021;44(2):137-71. [PubMed ID: 32725687]. https://doi.org/10.1111/jvp.12895.
-
5.
Jiang H, Cheng H, Liang Y, Yu S, Yu T, Fang J, et al. Diverse mobile genetic elements and conjugal transferability of sulfonamide resistance genes (sul1, sul2, and sul3) in escherichia coli isolates from penaeus vannamei and pork from large markets in Zhejiang, China. Front Microbiol. 2019;10:1787. [PubMed ID: 31428076]. [PubMed Central ID: PMC6690019]. https://doi.org/10.3389/fmicb.2019.01787.
-
6.
Nunes OC, Manaia CM, Kolvenbach BA, Corvini PF. Living with sulfonamides: a diverse range of mechanisms observed in bacteria. Appl Microbiol Biotechnol. 2020;104(24):10389-408. [PubMed ID: 33175245]. https://doi.org/10.1007/s00253-020-10982-5.
-
7.
Petrovska L, Mather AE, AbuOun M, Branchu P, Harris SR, Connor T, et al. Microevolution of monophasic Salmonella typhimurium during epidemic, United Kingdom, 2005-2010. Emerg Infect Dis. 2016;22(4):617-24. [PubMed ID: 26982594]. [PubMed Central ID: PMC4806966]. https://doi.org/10.3201/eid2204.150531.
-
8.
Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23(6):1089-97. [PubMed ID: 9106201]. https://doi.org/10.1046/j.1365-2958.1997.3101672.x.
-
9.
Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol. 2011;9(1):9-14. [PubMed ID: 21113180]. [PubMed Central ID: PMC3255095]. https://doi.org/10.1038/nrmicro2490.
-
10.
Hensel M. Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol. 2004;294(2-3):95-102. [PubMed ID: 15493819]. https://doi.org/10.1016/j.ijmm.2004.06.025.
-
11.
Sturenburg E, Kuhn A, Mack D, Laufs R. A novel extended-spectrum beta-lactamase CTX-M-23 with a P167T substitution in the active-site omega loop associated with ceftazidime resistance. J Antimicrob Chemother. 2004;54(2):406-9. [PubMed ID: 15201232]. https://doi.org/10.1093/jac/dkh334.
-
12.
Sonbol FI, Khalil MA, Mohamed AB, Ali SS. Correlation between antibiotic resistance and virulence of Pseudomonas aeruginosa clinical isolates. Turk J Med Sci. 2015;45(3):568-77. [PubMed ID: 26281322]. https://doi.org/10.3906/sag-1406-58.
-
13.
Pai H, Lyu S, Lee JH, Kim J, Kwon Y, Kim JW, et al. Survey of extended-spectrum beta-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J Clin Microbiol. 1999;37(6):1758-63. [PubMed ID: 10325320]. [PubMed Central ID: PMC84943]. https://doi.org/10.1128/JCM.37.6.1758-1763.1999.
-
14.
Doma AO, Popescu R, Mituletu M, Muntean D, Degi J, Boldea MV, et al. Comparative evaluation of qnrA, qnrB, and qnrS genes in Enterobacteriaceae ciprofloxacin-resistant cases, in swine units and a hospital from Western Romania. Antibiotics (Basel). 2020;9(10). [PubMed ID: 33066610]. [PubMed Central ID: PMC7602382]. https://doi.org/10.3390/antibiotics9100698.
-
15.
Yue M, Li X, Liu D, Hu X. Serotypes, antibiotic resistance, and virulence genes of Salmonella in children with diarrhea. Viral Hepat J. 2020;34(12). https://doi.org/10.1002/jcla.23525.
-
16.
Nikiema MEM, Kakou-Ngazoa S, Ky/Ba A, Sylla A, Bako E, Addablah AYA, et al. Characterization of virulence factors of Salmonella isolated from human stools and street food in urban areas of Burkina Faso. BMC Microbiol. 2021;21(1):338. [PubMed ID: 34895140]. [PubMed Central ID: PMC8665542]. https://doi.org/10.1186/s12866-021-02398-6.
-
17.
Wang W, Chen J, Shao X, Huang P, Zha J, Ye Y. Occurrence and antimicrobial resistance of Salmonella isolated from retail meats in Anhui, China. Food Sci Nutr. 2021;9(9):4701-10. [PubMed ID: 34531984]. [PubMed Central ID: PMC8441314]. https://doi.org/10.1002/fsn3.2266.
-
18.
Bhowmick PP, Devegowda D, Ruwandeepika HAD, Karunasagar I, Karunasagar I. Presence of Salmonella pathogenicity island 2 genes in seafood-associated Salmonella serovars and the role of the sseC gene in survival of Salmonella enterica serovar Weltevreden in epithelial cells. Microbiology (Reading). 2011;157(Pt 1):160-8. [PubMed ID: 20884689]. https://doi.org/10.1099/mic.0.043596-0.
-
19.
WHO global Salm-Surv strategic plan 2000-2005. World Health Organization; 2022. Available from: http://www.who.int/salmsurv/links/en/gssstrategyplanreport.pdf.
-
20.
WHO global Salm-Surv strategic plan 2006-2010. World Health Organization; 2022. Available from: https://www.who.int/salmsurv/general/documents/GSS_STRATEGICPLAN2006.
-
21.
Xu H, Zhang W, Zhang K, Zhang Y, Wang Z, Zhang W, et al. Characterization of Salmonella serotypes prevalent in asymptomatic people and patients. BMC Infect Dis. 2021;21(1):632. [PubMed ID: 34210275]. [PubMed Central ID: PMC8252320]. https://doi.org/10.1186/s12879-021-06340-z.
-
22.
Shen H, Chen H, Ou Y, Huang T, Chen S, Zhou L, et al. Prevalence, serotypes, and antimicrobial resistance of Salmonella isolates from patients with diarrhea in Shenzhen, China. BMC Microbiol. 2020;20(1):197. [PubMed ID: 32631309]. [PubMed Central ID: PMC7339465]. https://doi.org/10.1186/s12866-020-01886-5.
-
23.
O'Regan E, Quinn T, Frye JG, Pages JM, Porwollik S, Fedorka-Cray PJ, et al. Fitness costs and stability of a high-level ciprofloxacin resistance phenotype in Salmonella enterica serotype enteritidis: reduced infectivity associated with decreased expression of Salmonella pathogenicity island 1 genes. Antimicrob Agents Chemother. 2010;54(1):367-74. [PubMed ID: 19917752]. [PubMed Central ID: PMC2798541]. https://doi.org/10.1128/AAC.00801-09.
-
24.
Lou L, Zhang P, Piao R, Wang Y. Salmonella pathogenicity island 1 (SPI-1) and its complex regulatory network. Front Cell Infect Microbiol. 2019;9:270. [PubMed ID: 31428589]. [PubMed Central ID: PMC6689963]. https://doi.org/10.3389/fcimb.2019.00270.
-
25.
Karasova D, Sebkova A, Havlickova H, Sisak F, Volf J, Faldyna M, et al. Influence of 5 major Salmonella pathogenicity islands on NK cell depletion in mice infected with Salmonella enterica serovar Enteritidis. BMC Microbiol. 2010;10:75. [PubMed ID: 20226037]. [PubMed Central ID: PMC2848020]. https://doi.org/10.1186/1471-2180-10-75.
-
26.
Amaya FA, Blondel CJ, Barros-Infante MF, Rivera D, Moreno-Switt AI, Santiviago CA, et al. Identification of type VI secretion systems effector proteins that contribute to interbacterial competition in Salmonella Dublin. Front Microbiol. 2022;13:811932. [PubMed ID: 35222335]. [PubMed Central ID: PMC8867033]. https://doi.org/10.3389/fmicb.2022.811932.
-
27.
Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006;6:101. [PubMed ID: 17173674]. [PubMed Central ID: PMC1764016]. https://doi.org/10.1186/1471-2180-6-101.
-
28.
Fabrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26(2):308-41. [PubMed ID: 23554419]. [PubMed Central ID: PMC3623383]. https://doi.org/10.1128/CMR.00066-12.
-
29.
Xu F, Min F, Wang J, Luo Y, Huang S, Chen M, et al. Development and evaluation of a Luminex xTAG assay for sulfonamide resistance genes in Escherichia coli and Salmonella isolates. Mol Cell Probes. 2020;49:101476. [PubMed ID: 31678631]. https://doi.org/10.1016/j.mcp.2019.101476.
-
30.
Adesiji YO, Deekshit VK, Karunasagar I. Antimicrobial-resistant genes associated with Salmonella spp. isolated from human, poultry, and seafood sources. Food Sci Nutr. 2014;2(4):436-42. [PubMed ID: 25473501]. [PubMed Central ID: PMC4221842]. https://doi.org/10.1002/fsn3.119.
-
31.
Shitta G, Makanjuola O, Adefioye O, Olowe OA. Extended Spectrum Beta Lactamase (ESBL), blaTEM,blaSHV and blaCTX-M, resistance genes in community and healthcare associated Gram Negative bacteria from Osun State, Nigeria. Infect Disord Drug Targets. 2021;21(4):595-602. [PubMed ID: 32729432]. https://doi.org/10.2174/1871526520999200729181559.
