Abstract
Background:
Viral acute gastroenteritis (AGE) is a major cause of morbidity in childhood and leads to hospitalization in developed countries, such as Iran.Objectives:
The aim of this study was to determine the prevalence and viral types (rotavirus, adenovirus, human parechoviruses-1, and human bocavirus) of acute nonbacterial gastroenteritis in hospitalized children.Patients and Materials:
This was a across-sectional prospective study performed at the Pediatric Department of Rasoul Hospital, Tehran, Iran (2009-2011) on 80 hospitalized children with viral AGE. All Stool samples were collected on viral transport media. Human bocavirus (HBoV) was detected using the Real-time PCR TaqMan method. Molecular detection of human parechovirus type 1 (HPeV-1) RNA in stool samples was done using a specific nested reverse transcription PCR (RT-PCR). Rota and adeno virus antigens were sought by rapid chromatographic tests. P values less than 0.05 were considered statistically significant.Results:
Fever was determined in 47.5% of cases (38), nausea and vomiting in 42.5% (34), respiratory symptoms in 16.3% (13), abdominal pain in 76%. Duration of diarrhea was 1-30 days (mean = 6.3 + 4.3 days). No dehydration was observed in 43.5% of subjects, mild dehydration in 33.8%, moderate dehydration in 17.5% and severe dehydration in 5% of cases. Positive rotavirus was found in 48.8% of cases (39), adenovirus in 20% (16), HBoV in 8% (6) and HPeV-1 in 23.2% (19), and adeno and rotaviruses co-infection in 6% (4). The frequency of positive HBoV was significantly lower than adeno and rotaviruses infection (P value = 0.0001). Rotavirus was more frequent in males (P value = 0.003) and in young children (17.49 months vs. 21.44 months) [P value = 0.03, CI = -13.4, 5.5]. Rotavirus infection was related to the degree of dehydration (P value = 0.001) but was not related to the presence of vomiting or fever (P value > 0.5).Conclusions:
This study indicates that viral agents, especially rotavirus (48.8%), HPeV-1 (23.2%) and adenovirus (20%) are the most important causes for viral AGE in children while HBoV (8%) is infrequent during childhood. Determination of various viral pathogens of AGE is very important in planning diarrhea disease control strategies in our country where rotavirus vaccination in not routinely used.Keywords
1. Background
Acute diarrhea disease is the second cause of death among all infectious diseases in children younger than five years old, worldwide (1). Viral acute gastroenteritis (AGE) is a major cause of morbidity during childhood and leads to hospitalization even in developed countries (2, 3). Different types of viruses such as norovirus, rotavirus, astrovirus, adenovirus, enterovirus and parachovirus cause AGE diseases (4-7). Human bocavirus (HBoV) is a recently discovered virus of the family Parvoviridae, genus Bocavirus, as a new agent associated with respiratory tract infections (RTI) and AGE in children (8-10). The frequency of HBoV differs between countries (Canada 1.5%, Sweden 3.1%, Australia 5.6%, Japan 5.7%, Germany 10.3% and Korea 11.3%) but its prevalence in Iran is unknown (11-13). Human parechovirus (HPeVs), along with human enteroviruses are associated with gastrointestinal and respiratory infections, and occasionally with neonatal sepsis and meningitis. Human parechovirus epidemiology and disease associations are not fully understood (14, 15).
Baumgarte et al. (14) studied two groups of patients of all ages, during one full year, with acute enteritis. In a prospective study on a group of 499 none hospitalized patients; the virus detection rate was 1.6%. One virus-positive patient was detected in every 39 individuals. Positive samples occurred only in summer and autumn. Only one patient had accompanying respiratory symptoms. No association with travel or animal contact was found. All positive patients, but one, were younger than two years old, with a neutral gender ratio. In these children, the detection rate was 11.6% (7 out of every 60 children). The range of viral load was 3,170 to 503,377,290 copies per gram or milliliter of stool. One of the highest viral loads was seen in a control child without any symptoms at the time of testing. Phylogenetic analysis showed mainly contemporary human parechovirus type 1 (HPeV1) strains in our patients but also showed a separate new lineage of HPeV1 in evolutionary transition from the historical prototype strain. Moreover, a brand new sixth HPeV type was identified.
Full genome analysis of the two viruses revealed recombination between HPeV1 and HPeV3 in one prototype and HPeV6 and HPeV1 in another. HPeV seems very common in children younger than two years old and specific RT-PCR for HPeV should be included in enteritis screening in children (14). Harvala et al. (15) reported HPeV as a significant cause of severe sepsis and fever with central nervous system involvement in young infants. The frequency of HPeVs detection varied greatly by year, with the highest frequency (7.2%) noted in 2008 exceeding that of Entroviruses. Specific targeting of young infants by HPeV type 3 may reflect a difference in tissue tropism between virus types or a lack of maternal antibody protection in young infants' consequent to the recent emergence of HPeV (15). Many studies explained the etiologies of diarrhea in Iranian children admitted to hospitals (16, 17). Rotavirus is a common cause of viral AGE reported in Iranian children (18-20). Adenovirus and astrovirus were also obtained from stool of children with AGE in Iran (22-23). Knowledge about the viral etiologies of AGE is very important in planning diarrhea control strategies and future vaccine development in our country (14-18).
2. Objectives
The aim of this study was to determine the prevalence and viral types (rotavirus, adenovirus, HPeV1 and HBoV) of acute onset gastroenteritis in hospitalized children in Rasoul Akram Hospital (Tehran) during a two-year period (2009-2011).
3. Patients and Materials
This study was a prospective cross sectional study on 80 hospitalized patients afflicted with acute onset gastroenteritis in the pediatric ward of Rasoul Hospital (2009-2011), Tehran, Iran. This study was approved by the Ethics Committee of the Research Center of Pediatric Infectious Diseases at Iran University of Medical Sciences. Data collection was performed after obtaining parental consent; all children with AGE were selected. Their medical histories were obtained by interviews and trained physicians using standardized questionnaires performed the clinical examinations. For each case, the questionnaire was completed by an authorized physician, covering different aspects such as age, gender and other relevant demographic variables. Clinical manifestations included vomiting, duration and type of diarrhea, duration of hospitalization and finally the lab test results (stool direct exams, biochemical parameters, complete blood count, stool culture and direct viral test in stool).
Cases with bacterial AGE based on stool culture or stool direct smear or other known causes (except viral causes) for AGE, including enteral (parasites, toxins, antibiotic associated diarrhea, celiac inflammatory diseases, lactase deficiency etc.) or parenteral (urinary infection, pneumonia, otitis media, sinusitis etc.) were excluded from the study. Acute gastroenteritis cases were selected after excluding other causes, detailed above (exclusion criteria). Stool samples were processed to detect rota and adenoviruses. A rapid chromatographic test was performed with RFDA QUICK Rotavirus/Adenovirus combo (R-Bio pharm, Germany, N1002) according to the manufacturer’s instructions. At the same time, a part of the stool samples was collected on viral transport media.
Molecular detection of HPeV-1 in stool samples was done. For HPeV-1-, RNA was isolated from stool suspensions and cDNA was prepared and amplified using a specific nested reverse transcription PCR (RT-PCR). We also compared this RT-PCR method with a cell culture procedure. On the other hand, HBoV was detected using the Real-time PCR Taq Man method. The stool samples were diluted 1:10 in PBS and centrifuged. Supernatants were collected and frozen at -70°C until use. DNA was extracted using the High Pure Viral Nucleic Acid kit (Roche Diagnostics GmbH, Mannheim, Germany). Extracted DNA pellets were resuspended in 100 μL of pure warmed elution buffer and stored at -20°C until use. For Real Time PCR of HBoV DNA we used HBoV nonstructural gene sequence that is conserved in all types of HBoV. We used the Rotor Gene 6000 (Corbett Research, Australia) instrument for Real Time PCR test with hydrolyses TaqMan probe assay.
Human bocavirus was detected in extracted DNA by PCR amplification of a 81 bp fragment of the NS1 gene, and the amplification reaction mixture contained 5 μL sample DNA, 10 mL TaqMan universal PCR master mix (PE Applied Bio systems), 0.1 μL bovine serum albumin (20 mg/mL), 300 nmol/liter of each primer (Boca-forward, AGAGGCTCGGGCTCATATCA; Boca-reverse, CACTTGGTCTGAGGTCTTCGAA), and 150 nmol/liter of the Boca probe (FAM- AGGACCACCCAATCARCCACCTATCGTCT-TAMRA, where FAM is 6-carboxyfluorescein and TAMRA is 6–carboxytetramethylrhodamine). Amplification was performed under standard amplification conditions in the following manner: 95°C for 10 minutes and 42 cycles of 95°C for 15 seconds and 60°C for 1 minute (9). HBY positive and negative samples were confirmed. HBY positive and negative samples were confirmed.
For statistical analysis, the student’s t test was used for continuous variables. The mann-whitney test and the chi-square or fisher's exact tests were used to compare groups. P-values less than 0.05 were considered statistically significant. The analyses were performed using the SPSS software (Version 11.5).
4. Results
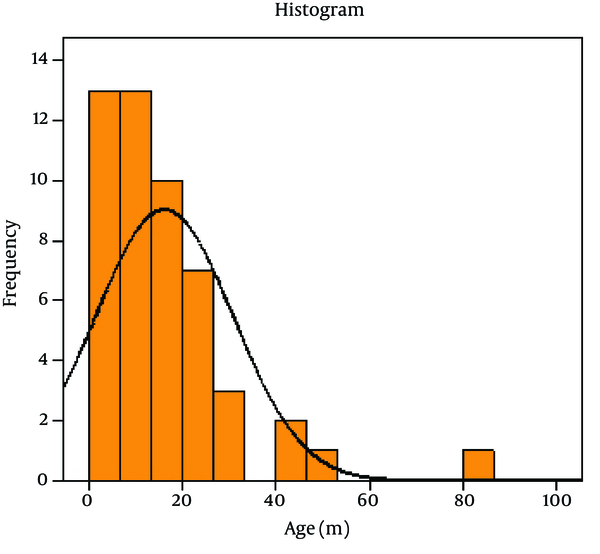
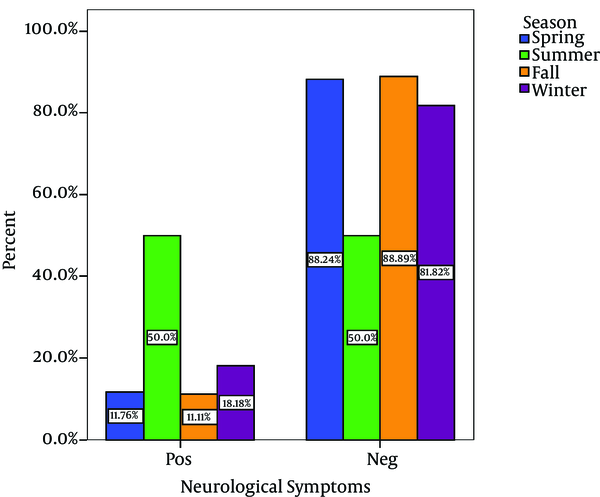
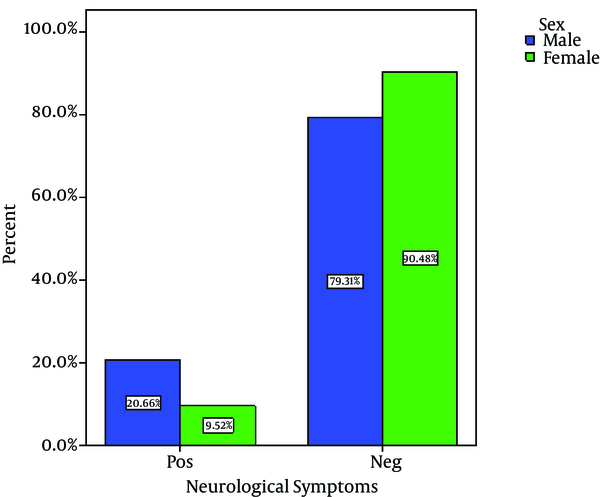
Out of 80 Children with viral gastroenteritis, 58% were male and 42% were female; aged between 2 and 108 months old (mean age of 19.51 + 21.2 months). The rate of viral AGE for different seasons was as follows; winter, 44%; spring, 34%, autumn, 18% and summer, 4% (Figure 1-3) 1-3) Fever was reported in 47.5% of cases, abdominal pain in 76%, nausea and vomiting in 42.5%, and respiratory symptoms in 16.3% (12). Duration of diarrhea was 1-30 days (mean = 6.3 + 4.3 days). No dehydration was detected in 43.5% of cases, mild dehydration in 33.8%, moderate dehydration in 17.5%, and severe dehydration in 5%. Positive rotavirus, adenovirus, HBoV and HPeV-1 was reported in 48.8% (39), 20% (16), 8% (6) and 23.2% (19) of cases, respectively. Adeno and rota virus co-infection was observed in 6% (4) of cases. Positive rota virus was higher in males (P value = 0.003) and in young children (17.49 months vs. 21.44 months) [P value = 0.03, CI = -13.4, 5.5]. Rotavirus infection was related to the degree of dehydration (P value = 0.001) but was not related to the presence of vomiting or fever (P value > 0.5). HPeV-1 infection (23.7%) was more frequent in male cases (P value < 0.001 and young children (< 1 year) [P value = 0.036], and during spring and autumn (P value < 0.001).
Age Histogram of Cases
Seasonal Distribution of Cases With Neurologic Symptoms
Comparision of Neurologic Symptoms Between 2 Sexes
5. Discussion
We studied 80 children (aged between 2 and 108 months, mean age: 19.51 + 21.2 months) with nonbacterial AGE. Most cases (78%) were admitted during winter and spring. Duration of diarrhea was 1-30 days (mean = 6.3 + 4.3 days). Fever was reported in 47.5% of cases, abdominal pain in 76%, vomiting in 42.5% and respiratory symptoms in 16.3%. Although no dehydration was reported in 43.5% of children, yet mild, moderate and severe dehydration was observed in 33.8%, 17.5% and 5% of cases respectively. Rotavirus, adenovirus, HBoV, HPeV-1 infection, adeno and rota co-infection was found in 48.8%, 20%, 8%, 23.2% and 6% of cases, respectively. The frequency of positive HBoV was significantly lower than adeno and rotavirus infections (P value = 0.0001).
The findings of this study were similar to that of a Korean study; 44.7% of all AGE cases were viral, including 25.7% rotavirus infection, 13.7% norovirus, 3.0% adenovirus, 1.1% astrovirus and 0.8% HBoV (9). In this study, similar to other Iranian studies rotavirus (48.8%) was the most common cause of viral AGE (19-20), especially in young (17.49 months) and male patients. Severe dehydration was frequent in cases with rotavirus GE but vomiting or fever was unusual findings. Esteghamati et al. (20) reported rotavirus infection in 59.1% of all diarrheal cases (n = 1298). Similar to our study, 85% of rotavirus infections occurred in young children (< 2 years) with peak prevalence during the cold season (September through to January). Furthermore, 30.9% of strains (110 positive rotavirus samples) had the G4P genotype (21). Zarnani et al. (18) reported 15.3% rotavirus infection amongst cases with AGE. Also, Kazemi et al. (19) detected rotavirus infection in 30.8% of AGE hospitalized cases and 12.1% of individuals from the control group (P value < 0.05).
Adenovirus was found in 20% of AGE cases, which is very similar to that detected by the present study. Most Iranian studies reported that the peak of viral gastroenteritis diseases is during the cold season (18-22). However, adenovirus infection was more common (20%) in our study than 2 other Iranian studies (21, 22). This higher adenovirus infection might be due to the precise selection of cases with true viral AGE or the use of different methods. Saderi et al. (21) reported 6.7% for enteric adenovirus and 2% for nonenteric adenovirus while Modarres et al. (22) detected adenoviruses infection in 2.6% of AGE cases and none of the healthy controls with a higher peak (48.1%) during winter. The rates of rotavirus, adenovirus and astrovirus AGE as reported by the Harvala et al. (15) study was 62%, 2.3%, and 3%, respectively. Most astrovirus (8.3%) and adenovirus (3.5%) infections were observed in children between 2 and 5 years old (22).
Human bocavirus infection has not yet been reported in the Iranian population. Human bocavirus prevalence in our study was 8%, which was significantly lower than adeno and rotaviruses (P value = 0.0001) and was more frequent than earlier reports from Canada (1.5%), Sweden (3.1%), Australia (5.6%) and Japan (5.7%) yet lower than other reports from Germany (10.3%) and Korea (11.3%) (10). The range of HBoV seroprevalence varies from 48% to 85% at the age of four years. Its peak is during the winter season and is often found to coincide with other pathogens (23). Lee et al. (9) detected HBoV in 0.8% of AGE cases, which suggests that it might play a minor role in gastroenteritis. Lau et al. (13) detected HBoV in 30 (2.1%) out of 1435 fecal samples. Fever and watery diarrhea were the most common symptoms. Co-infection with other pathogens occurred in 33% and 56% of respiratory and fecal samples, with minimal sequence variations. Recent data suggests that HBoV infections occur early in life and the virus replicates in the human gut (8, 9, 23). Some authors reported HBoV as an important agent for outbreaks of gastroenteritis in day care facilities for children (9, 10). Here, we found HPeV-1 positivity in 23.7% of cases. Most HPeV-1 positive cases were young (< 1 year) and males, and were admitted to the hospital during spring and autumn (detection of HPeV-1 was higher than the Baumgarte et al. study (1.6%)(14)). However, it is important to mention that they performed their study on non-hospitalized patients (14). Similar to our study, Baumgarte et al. (14) reported that 11.6% of HPeV-1 infections occurred in young cases (< 2 years).
5.1. Strengths of the Study
The higher rate of viral infection (in comparison with previous Iranian trials) might be due to precise selection of cases with true viral AGE, clear–cut exclusion criteria for other enteral or parenteral causes of AGE, or use of different methods for searching the etiological organisms, especially new viruses (HPeV-1 and HBoV) in our study. Human bocavirus had a lower prevalence in our study in comparison with adeno and rotaviruses (P value = 0.0001).
5.2. Limitations of the Study
The limited number of true nonbacterial AGE cases and diversity in methods for detection of viral infections were the major limitations. Although HBoV and HPeV-1 were found in 8% and 23.2% of cases yet probable viral co-infection such as norovirus and astrovirus were not evaluated here. Further studies are required to determine the role of other viral infections in diarrhea. This study indicates that viral agents, especially rotavirus (48.8%), HPeV-1 (23.2%) and adenovirus (20%) are the most important causes of viral AGE in children but HBoV (8%) is infrequent. Determination of various viral pathogens of AGE is very important in planning diarrhea disease control strategies in our country where rotavirus vaccination is not routinely used. Due to the presence of a safe and effective rotavirus vaccine, we suggest the use of rotavirus vaccination as a public health priority in Iran.
References
-
1.
Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35-40. [PubMed ID: 17204802]. https://doi.org/10.1136/bmj.39036.406169.80.
-
2.
Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201(11):1617-24. [PubMed ID: 20402596]. https://doi.org/10.1086/652403.
-
3.
Ito H, Otabe O, Katsumi Y, Matsui F, Kidowaki S, Mibayashi A, et al. The incidence and direct medical cost of hospitalization due to rotavirus gastroenteritis in Kyoto, Japan, as estimated from a retrospective hospital study. Vaccine. 2011;29(44):7807-10. [PubMed ID: 21821087]. https://doi.org/10.1016/j.vaccine.2011.07.105.
-
4.
Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68(2):171-7. [PubMed ID: 1694734].
-
5.
Weinstein M. Seizures and encephalopathy as the presenting sign of viral gastroenteritis. Pediatr Emerg Care. 2006;22(8):579-81. [PubMed ID: 16912627]. https://doi.org/10.1097/01.pec.0000230703.66399.2e.
-
6.
Li D, Gu AZ, Zeng SY, Yang W, He M, Shi HC. Monitoring and evaluation of infectious rotaviruses in various wastewater effluents and receiving waters revealed correlation and seasonal pattern of occurrences. J Appl Microbiol. 2011;110(5):1129-37. [PubMed ID: 21276144]. https://doi.org/10.1111/j.1365-2672.2011.04954.x.
-
7.
Narci H, Ugur M, Kisinma A, Turan H. Age distribution and seasonal pattern of rotavirus infection in children under 5 years. Jundishapur J Microbiol. 2013;6(1):16-9. https://doi.org/10.5812/jjm.4626.
-
8.
Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43(3):283-8. [PubMed ID: 16804840]. https://doi.org/10.1086/505399.
-
9.
Lee JI, Chung JY, Han TH, Song MO, Hwang ES. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J Infect Dis. 2007;196(7):994-7. [PubMed ID: 17763319]. https://doi.org/10.1086/521366.
-
10.
Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, et al. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194(9):1283-90. [PubMed ID: 17041855]. https://doi.org/10.1086/508219.
-
11.
Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, et al. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194(9):1276-82. [PubMed ID: 17041854]. https://doi.org/10.1086/508213.
-
12.
Bastien N, Brandt K, Dust K, Ward D, Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis. 2006;12(5):848-50. [PubMed ID: 16704852]. https://doi.org/10.3201/eid1205.051424.
-
13.
Lau SK, Yip CC, Que TL, Lee RA, Au-Yeung RK, Zhou B, et al. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis. 2007;196(7):986-93. [PubMed ID: 17763318]. https://doi.org/10.1086/521310.
-
14.
Baumgarte S, de Souza Luna LK, Grywna K, Panning M, Drexler JF, Karsten C, et al. Prevalence, types, and RNA concentrations of human parechoviruses, including a sixth parechovirus type, in stool samples from patients with acute enteritis. J Clin Microbiol. 2008;46(1):242-8. [PubMed ID: 18057123]. https://doi.org/10.1128/JCM.01468-07.
-
15.
Harvala H, Robertson I, Chieochansin T, McWilliam Leitch EC, Templeton K, Simmonds P. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J Infect Dis. 2009;199(12):1753-60. [PubMed ID: 19456229]. https://doi.org/10.1086/599094.
-
16.
Kermani NA, Jafari F, Mojarad HN, Hoseinkhan N, Zali R. Prevalence and associated factors of persistent diarrhoea in Iranian children admitted to a paediatric hospital. East Mediterr Health J. 2010;16(8):831-6. [PubMed ID: 21469564].
-
17.
Jafari F, Garcia-Gil LJ, Salmanzadeh-Ahrabi S, Shokrzadeh L, Aslani MM, Pourhoseingholi MA, et al. Diagnosis and prevalence of enteropathogenic bacteria in children less than 5 years of age with acute diarrhea in Tehran children's hospitals. J Infect. 2009;58(1):21-7. [PubMed ID: 19117609]. https://doi.org/10.1016/j.jinf.2008.10.013.
-
18.
Zarnani AH, Modarres S, Jadali F, Sabahi F, Moazzeni SM, Vazirian F. Role of rotaviruses in children with acute diarrhea in Tehran, Iran. J Clin Virol. 2004;29(3):189-93. [PubMed ID: 14962788]. https://doi.org/10.1016/S1386-6532(03)00123-9.
-
19.
Kazemi A, Tabatabaie F, Agha Ghazvini MR, Kelishadi R. The role of rotavirus in acute pediatric diarrhea in Isfahan, Iran. Pak J Med Sci. 2006;22(3):282-5.
-
20.
Eesteghamati A, Gouya M, Keshtkar A, Najafi L, Zali MR, Sanaei M, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in iran. J Infect Dis. 2009;200 Suppl 1:S244-7. [PubMed ID: 19821714]. https://doi.org/10.1086/605050.
-
21.
Saderi H, Roustai MH, Sabahi F, Sadeghizadeh M, Owlia P, De Jong JC. Incidence of enteric adenovirus gastroenteritis in Iranian children. Clin Diagn Virol. 2002;24(1):1-5. https://doi.org/10.1016/S1386-6532(01)00206-2.
-
22.
Modarres S, Jam Afzon F, Modarres S. Enteric adenovirus infection in infants and young children with acute gastroenteritis in Tehran. Acta Med Iran. 2006;44(5):349-53.
-
23.
Albuquerque MC, Rocha LN, Benati FJ, Soares CC, Maranhao AG, Ramirez ML, et al. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg Infect Dis. 2007;13(11):1756-8. [PubMed ID: 18217564]. https://doi.org/10.3201/eid1311.070671.