Abstract
Background:
Bovine viral diarrhea (BVD) is an economically important disease of cattle distributed worldwide. Diagnosis of BVD relies on laboratory-based detection of its viral causing agent or virus specific antibodies and the most common laboratory method for this purpose is Enzyme-Linked Immunosorbent Assay (ELISA).Objectives:
The current study was aimed to develop a simple indirect ELISA to detect antibodies against Bovine Viral Diarrhea Virus (BVDV) in the sera of infected cattle.Materials and Methods:
A new simple indirect ELISA method was developed to detect BVDV infection by prokaryotically (Escherichia coli, BL21 strain) expressed recombinant whole nonstructural protein 3 (NS3) of BVDV (NADL strain). Four hundred bovine serum samples were evaluated by the newly developed NS3-ELISA and virus neutralization test (VNT) as the gold standard method to diagnose BVD. Among these samples, 289 sera had been previously tested by a commercial ELISA kit.Results:
Statistical analyses showed a very high correlation between the results of the developed NS3-ELISA and VNT (kappa coefficient = 0.935, P < 0.001), with the relative sensitivity and specificity of 94% and 98.8%, respectively. There was also a high correlation between the results of NS3-ELISA and the commercial ELISA kit (kappa coefficient = 0.802, P < 0.001) with the relative sensitivity and specificity of 90.72% and 91.15%, respectively.Conclusions:
The newly developed simple indirect ELISA showed high sensitivity and specificity with respect to VNT. Developing such a simple, sensitive, and specific ELISA which is much less expensive than the available commercial ELISA kits can improve the detection of BVDV infections, help to eliminate the disease from herds, and decrease economic losses caused by this disease.Keywords
Bovine Viral Diarrhea Bovine Viral Diarrhea Virus ELISA Nonstructural Protein 3 NS3
1. Background
Bovine Viral Diarrhea (BVD) is one of the most important cattle diseases characterized by diarrhea, abortion, stillbirth, return to estrus, and milk drop. Economic impact of BVD has led a number of countries in Europe to start eradication or control programs (1, 2). BVD is caused by Bovine Viral Diarrhea Virus (BVDV), which belongs to Pestivirus genus, Flaviviridae family. Diagnosis of BVD relies on laboratory-based detection of its viral causing agent or virus specific antibodies. Although virus neutralization test (VNT) is the gold standard method to detect the virus specific antibodies (3), Enzyme-Linked Immunosorbent Assay (ELISA) is a popular laboratory method for this purpose (4).
The most immunogenic proteins of BVDV (NADL strain) are Erns, E2, and nonstructural protein 3 (NS3) (5). NS3 molecule is an 80 kDa protein which is highly conserved among Pestiviruses. This protein, containing a serine protease domain at N-terminal and a domain with RNA helicase activity at C-terminal (6-8), is essential for pestivirus RNA replication (9). NS3 is strongly recognized by polyclonal convalescent sera (10). Animals vaccinated with modified live vaccines also have a strong antibody response to this protein (11). Antibodies to NS3 do not neutralize the virus infectivity; however, these antibodies can be readily detected by other serological tests. Therefore, NS3 is an important antigen in BVDV serology (4) and is suggested as a proper candidate antigen to detect antibodies against the virus. The current study used a prokaryotically expressed (Escherichia coli, BL strain) NS3 fusion protein as an ELISA antigen to develop a simple indirect ELISA for the detection of antibodies against BVDV in the sera of infected cattle.
2. Objectives
The current study aimed to develop a simple indirect ELISA to detect antibodies against BVDV in the sera of infected cattle.
3. Materials and Methods
3.1. Preparation of ELISA Antigen
Full-length cDNA coding region of the whole NS3 molecule (nucleotide 5423 to 7471, 2049 base pair, 683 residues) was cloned into pMAL-c2X expression vector at the XbaI/PstI sites. The recombinant maltose binding protein (MBP)-NS3 fusion protein was expressed in E. coli (BL21 strain) followed by purification of the protein with an amylose-resin column chromatography as previously described (12).
3.2. Bovine Serum Samples
Four hundred bovine serum samples were randomly collected from slaughtered cattle of both sexes and different ages at the abattoir. All serum samples were heat inactivated at 56°C for 30 minutes. Among these samples, 289 sera had been previously examined by a commercial ELISA kit (IDEXX, USA) to detect the anti-BVDV antibodies.
3.3. Virus Neutralization Test
All bovine sera were duplicately tested for the presence of virus neutralizing antibodies against BVDV according to the standard microtitration procedure described in the OIE (World Organization for Animal Health) manual (13). Briefly, 50 μL of 1:5 dilution of each serum sample was added into two wells of a 96-wells cell culture microplate; thereafter, 50 μL (400 TCID50) of BVDV-1 (cytopathic NADL strain) was added into each well and the plate was incubated at 37°C for one hour in a humidified CO2 incubator. Finally, 1.5 × 104 BT cells were added to each well and the micro plate was incubated at 37°C for five days. The plate was observed daily for the presence of virus cytopathic effects. Four wells were allocated to each of the cell and virus controls and the results were documented compared to these control.
3.4. Indirect ELISA Procedure
The optimum concentration of ELISA antigen, serum dilution, and conjugated anti-antibody dilution were determined using checkerboard titration method. The amylose-resin purified recombinant MBP-NS3 fusion protein, at 300 ng/well, was applied into the 96-well polystyrene microtiter plates (Karizmehr Co., Iran) as ELISA antigen and incubated for 16 hours at 4°C. All the subsequent incubations were at room temperature and the plates were washed four times with PBST (PBS containing 0.05% Tween 20) after each step. Blocking of the unreacted sites was carried out for three hours using PBS containing 0.2% Tween 20. After washing the plate, a 1/400 dilution of the bovine serum samples, in PBST containing 10% chicken serum, were added to the wells and incubated for 30 minutes.
The serum diluent buffer also contained 5% of soluble proteins of IPTG (Isopropyl β-D-1-thiogalactopyranoside) induced E. coli cells containing pMAL-c2X plasmid and purified MBP at a final concentration of 1.2 mg/L. After a washing step, a 1/1300 dilution of rabbit anti-bovine IgG-HRP (Sigma, USA) in PBST containing 1% rabbit serum was added to the wells and incubated for one hour. The ELISA plate was washed again with PBST and tetramethylbenzidine substrate solution was added to the wells and incubated for 10 minutes. The reactions were then stopped by addition of 0.1 M HCl and finally, optical density (OD) values were measured at 450 nm with a plate reader (Pishtazteb Co., Iran).
3.5. Statistical Analyses
Receiver Operating Characteristic Curve (ROC) was used to determine the cut-off value of the MBP-NS3-ELISA, considering the maximum correlation between the results of VNT and MBP-NS3-ELISA. Thereafter, relative sensitivity and specificity of MBP-NS3-ELISA compared to VNT and the commercial ELISA kit were calculated and analyzed statistically.
4. Results
4.1. Preparation of ELISA Antigen
Recombinant MBP-NS3 protein was purified by amylose-resin column chromatography as shown in the Figure 1. This recombinant protein was expressed as a fusion protein of about 117 kDa with MBP (42.5 kDa) at the N-terminal part.
Purification of the Recombinant MBP-NS3 Protein

4.2. Virus Neutralization Test
Each bovine serum sample was tested in duplicate. The results of VNT for all sera were summarized in Table 1.
The Results of VNT
| Result | No. (%) |
|---|---|
| Negative | 250 (62.5) |
| Positive | 150 (37.5) |
| Total | 400 (100) |
4.3. ELISA and Statistical Analyses
Considering the maximum correlation between the results of MBP-NS3-ELISA and VNT as the gold standard method for serodiagnosis of BVDV infection, the calculated cut-off value by ROC was 0.303. While 150 serum samples were positive with VNT, and 144 serum samples were positive by MBP-NS3-ELISA. On the other hand, 250 serum samples negative for VNT, whereas 256 of all samples were negative for MBP-NS3-ELISA. A visual comparison of the results of these two methods is shown in Figure 2. McNemar’s test indicated no significant difference between the results of the two methods (P > 0.05). Kappa coefficient showed a very high correlation between these two methods (Kappa coefficient = 0.935, P < 0.001). The relative sensitivity and specificity of MBP-NS3-ELISA with respect to VNT were 94% and 98.8%, respectively and the accuracy of the developed MBP-NS3-ELISA was 97%. Quantitative comparison of the VNT and MBP-NS3-ELISA results is summarized in Table 2.
The results of 289 serum samples previously tested with commercial ELISA kit were compared to the results of MBP-NS3-ELISA. While 97 serum samples were positive with commercial ELISA kit, 105 serum samples were positive by MBP-NS3-ELISA. On the other hand, 192 serum samples were negative with commercial ELISA kit, whereas 184 of all samples were negative for MBP-NS3-ELISA. A visual comparison of the results of these two methods is shown in Figure 3. McNemar’s test indicated no significant difference between the results of the two methods (P > 0.05). Kappa coefficient showed a high correlation between these two methods (Kappa coefficient = 0.802, P < 0.001). The relative sensitivity and specificity of MBP-NS3-ELISA with respect to commercial ELISA kit were 90.72% and 91.15%, respectively; the accuracy of the developed MBP-NS3-ELISA was 91%. Quantitative comparison of the commercial ELISA kit and MBP-NS3-ELISA results is summarized in Table 3.
The Comparison of MBP-NS3-ELISA and Virus Neutralization Test Results
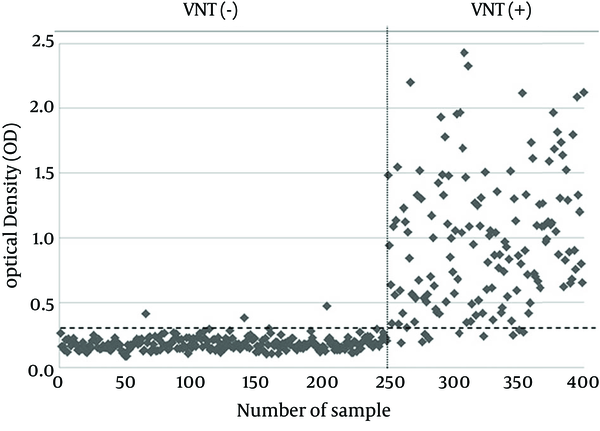
The Comparison of MBP-NS3-ELISA and Virus Neutralization Test Results
| Variables | Values | ||
|---|---|---|---|
| + | − | Total | |
| VNT NS3-ELISA | |||
| + | 141 | 3 | 144 |
| − | 9 | 247 | 256 |
| Total | 150 | 250 | 400 |
The Comparison of MBP-NS3-ELISA and Commercial ELISA Kit Results

The Comparison of MBP-NS3-ELISA and Commercial ELISA Kit Results
| Variables | Values | ||
|---|---|---|---|
| + | − | Total | |
| Kit NS3-ELISA | |||
| + | 88 | 17 | 105 |
| − | 9 | 175 | 184 |
| Total | 97 | 192 | 289 |
5. Discussion
The first step to control eradicate BVD is to eliminate persistently infected (PI) animals and determine the prevalence of antibodies against the viral causal agent in order to monitor the virus circulation. The first generations of diagnostic ELISA kits utilized the extracts of virus infected cell cultures as ELISA detector antigens (14, 15). Several ELISAs have been recently developed to detect BVDV infections using recombinant NS3 protein (16-19). Eukaryotic or prokaryotic recombinant NS3 is used for this purpose and this protein has shown high sensitivity and specificity to detect BVDV infection in comparison with whole virus antigen (16, 20). Thus, production of a recombinant form of NS3 in large amounts is economically very important and can be very useful to manufacture BVDV antibody ELISA kits. Although it is shown that eukaryotic expression of NS3 increases sensitivity and specificity of ELISA kits (18), prokaryotic expression of NS3 is still considered, since it is simple and less expensive than eukaryotic expression of the protein.
So far, several ELISAs are developed using prokaryotically expressed NS3 to detect anti-BVDV antibodies. Reddy et al. (17) cloned a 917-bp segment of NS3 (p80) into pGex-2T plasmid vector containing the glutathione-S-transferase (GST) gene and the recombinant protein was expressed in E. coli and used as an ELISA antigen to detect anti-BVDV antibodies. A 1152-bp cDNA fragment of NS3 (2/3rd of NS3 gene from C-terminal) was cloned into pGEMT Easy Vector and expressed in E. coli by Bhatia et al. (21) and used as a detector ELISA antigen in their developed competitive inhibition ELISA (CI-ELISA) using a monoclonal antibody. Lecomte et al. (16) used a recombinant 80 kDa antigen of the BVDV/Osloss virus strain as a fusion protein with β-galactosidase to detect BVDV specific antibodies by ELISA. They also developed a competitive ELISA which was more specific than the direct assay.
Vanderheijden et al. (18) inserted a 2183-nucleotide fragment containing encoding sequence of p80 (Osloss strain) into the pARHS3 plasmid and analyzed the expressed protein by a competitive ELISA. Although the prokaryotic expression of recombinant NS3 is a simple and inexpensive method, compared to the eukaryotic expression, researchers tried to develop competitive ELISAs by monoclonal antibodies and/or eukaryotically expressed recombinant NS3 molecule to increase sensitivity and specificity of the assay. In fact, one of the major problems of prokaryotic expression of NS3 molecule, particularly whole NS3 molecule, is that it is a large insoluble protein which aggregates as inclusion bodies in the bacterial host cells. Thus, researchers had to use high concentration of urea to solubilize the recombinant NS3 protein (inclusion bodies) and then renature it by gradient dialysis of the solution and it is obviously a time-consuming and expensive process. This problem was solved by another plasmid vector, pMAL-c2X, to clone and express the recombinant NS3 molecule used in this study. This vector encodes a maltose binding protein at the N-terminal part of the molecule which is a soluble protein and can even solubilize the recombinant protein expressed with it as a fusion protein (22, 23).
MBP tag makes it possible to purify the recombinant protein by amylose-resin. In addition, with the enhanced solubility of the expressed recombinant NS3, there was no need to treat the recombinant protein with urea for the purification. The current study developed an indirect ELISA using the recombinant MBP-NS3 molecule as an ELISA detector antigen. The protein was expressed in E. coli in a simple and inexpensive manner and it was sufficiently soluble to be purified without any treatment. MBP-NS3 based ELISA showed a high degree of sensitivity and specificity in comparison with the results of VNT; kappa coefficients indicated a very high correlation between MBP-NS3-ELISA with VNT (kappa = 0.935, P < 0.001) and the commercial ELISA kit (kappa = 0.802, P < 0.001), although there were some differences.
Nine out of 150 positive samples by VNT were negative by MBP-NS3-ELISA. This could be due to 1) a recent infection which results in the production of IgM; therefore, the OD values of such serum samples may decrease significantly causing the samples to be determined as negative by MBP-NS3-ELISA, 2) presence of non-specific inhibitor factors in the tested serum samples that prevented virus propagation in the cell culture, and 3) vaccination of animals with an inactive vaccine that induces the production of neutralizing antibodies but not antibodies against BVDV NS3 protein. On the other hand, 3 VNT negative samples were positive by MBP-NS3-ELISA. This could also be due to the strain of the employed virus in VNT and/or the amount (TCID50) of the employed virus in VNT. In this study, maximum recommended TCID50 of the virus (400 TCID50) was utilized in VNT. So, it was more stringent and this could decrease the sensitivity and increase the specificity of the assay. The differences between the results of MBP-NS3-ELISA and the commercial ELISA kit probably resulted from the type of employed ELISA detector antigen(s) or standardization of the procedures.
Meanwhile, the developed ELISA is a simple ELISA which has no need to monoclonal antibodies to increase sensitivity or specificity of the assay. Statistical analyses showed that this developed ELISA is highly sensitive and specific in comparison with the viral neutralization test, which is the reference test for the serological diagnosis of BVDV. Developing such a simple, sensitive, and specific ELISA which is much less expensive than the available commercial ELISA kits can improve the detection of BVDV infections, help to eliminate the disease from herds, and decrease the economic losses caused by this disease. The current study developed a new simple indirect ELISA with a prokaryotically expressed recombinant whole NS3 molecule as ELISA antigen to detect anti-BVDV antibodies in sera of the infected cattle. The developed ELISA showed high sensitivity and specificity with respect to VNT.
Acknowledgements
References
-
1.
Greiser-Wilke I, Grummer B, Moennig V. Bovine viral diarrhoea eradication and control programmes in Europe. Biologicals. 2003;31(2):113-8. https://doi.org/10.1016/s1045-1056(03)00025-3.
-
2.
Presi P, Heim D. BVD eradication in Switzerland--a new approach. Vet Microbiol. 2010;142(1-2):137-42. [PubMed ID: 19883982]. https://doi.org/10.1016/j.vetmic.2009.09.054.
-
3.
Edwards S. The diagnosis of bovine virus diarrhoea-mucosal disease in cattle. Rev Sci Tech. 1990;9(1):115-30. [PubMed ID: 1966717].
-
4.
Sandvik T. Laboratory diagnostic investigations for bovine viral diarrhoea virus infections in cattle. Vet Microbiol. 1999;64(2-3):123-34. [PubMed ID: 10028167].
-
5.
Collett MS. Molecular genetics of pestiviruses. Comp Immunol Microbiol Infect Dis. 1992;15(3):145-54. [PubMed ID: 1325329].
-
6.
Collett MS, Wiskerchen M, Welniak E, Belzer SK. Bovine viral diarrhea virus genomic organization. Arch Virol Suppl. 1991;3:19-27. [PubMed ID: 9210922].
-
7.
Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17(12):4713-30. [PubMed ID: 2546125].
-
8.
Wiskerchen M, Collett MS. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology. 1991;184(1):341-50. [PubMed ID: 1651596].
-
9.
Agapov EV, Murray CL, Frolov I, Qu L, Myers TM, Rice CM. Uncleaved NS2-3 is required for production of infectious bovine viral diarrhea virus. J Virol. 2004;78(5):2414-25. [PubMed ID: 14963137].
-
10.
Donis RO, Corapi WV, Dubovi EJ. Bovine viral diarrhea virus proteins and their antigenic analyses. Arch Virol Suppl. 1991;3:29-40. [PubMed ID: 9210923].
-
11.
Bolin SR, Ridpath JF. Specificity of neutralizing and precipitating antibodies induced in healthy calves by monovalent modified-live bovine viral diarrhea virus vaccines. Am J Vet Res. 1989;50(6):817-21. [PubMed ID: 2548419].
-
12.
Mahmoodi P, Shapouri MR, Ghorbanpour M, Ekhtelat M, Hajikolaei MR, Lotfi M, et al. Epitope mapping of bovine viral diarrhea virus nonstructural protein 3. Vet Immunol Immunopathol. 2014;161(3-4):232-9. [PubMed ID: 25205011]. https://doi.org/10.1016/j.vetimm.2014.08.012.
-
13.
OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (mammals, birds and bees). 2008.
-
14.
Howard CJ, Clarke MC, Brownlie J. An Enzyme-Linked Immunosorbent Assay (ELISA) for the detection of antibodies to Bovine Viral Diarrhoea Virus (BVDV) in cattle sera. Veterin Microbiol. 1985;10(4):359-69. https://doi.org/10.1016/0378-1135(85)90006-9.
-
15.
Justewicz DM, Magar R, Marsolais G, Lecomte J. Bovine viral diarrhea virus-infected MDBK monolayer as antigen in enzyme-linked immunosorbent assay (ELISA) for the measurement of antibodies in bovine sera. Vet Immunol Immunopathol. 1987;14(4):377-84. [PubMed ID: 3037768].
-
16.
Lecomte C, Pin JJ, De Moerlooze L, Vandenbergh D, Lambert AF, Pastoret PP, et al. ELISA detection of bovine viral diarrhoea virus specific antibodies using recombinant antigen and monoclonal antibodies. Vet Microbiol. 1990;23(1-4):193-201. [PubMed ID: 2169672].
-
17.
Reddy JR, Kwang J, Okwumabua O, Kapil S, Loughin TM, Lechtenberg KF, et al. Application of recombinant bovine viral diarrhea virus proteins in the diagnosis of bovine viral diarrhea infection in cattle. Vet Microbiol. 1997;57(2-3):119-33. [PubMed ID: 9355247].
-
18.
Vanderheijden N, De Moerlooze L, Vandenbergh D, Chappuis G, Renard A, Lecomte C. Expression of the bovine viral diarrhoea virus Osloss p80 protein: its use as ELISA antigen for cattle serum antibody detection. J Gen Virol. 1993;74 ( Pt 7):1427-31. [PubMed ID: 8393083].
-
19.
Chimeno Zoth S, Taboga O. Multiple recombinant ELISA for the detection of bovine viral diarrhoea virus antibodies in cattle sera. J Virol Methods. 2006;138(1-2):99-108. [PubMed ID: 16963129]. https://doi.org/10.1016/j.jviromet.2006.07.025.
-
20.
Beaudeau F, Belloc C, Seegers H, Assie S, Sellal E, Joly A. Evaluation of a blocking ELISA for the detection of bovine viral diarrhoea virus (BVDV) antibodies in serum and milk. Vet Microbiol. 2001;80(4):329-37. [PubMed ID: 11348769].
-
21.
Bhatia S, Sood R, Mishra N, Pattnaik B, Pradhan HK. Development and evaluation of a MAb based competitive-ELISA using helicase domain of NS3 protein for sero-diagnosis of bovine viral diarrhea in cattle and buffaloes. Res Vet Sci. 2008;85(1):39-45. [PubMed ID: 17983635]. https://doi.org/10.1016/j.rvsc.2007.09.013.
-
22.
Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8(8):1668-74. [PubMed ID: 10452611]. https://doi.org/10.1110/ps.8.8.1668.
-
23.
Sachdev D, Chirgwin JM. Solubility of proteins isolated from inclusion bodies is enhanced by fusion to maltose-binding protein or thioredoxin. Protein Expr Purif. 1998;12(1):122-32. [PubMed ID: 9473466]. https://doi.org/10.1006/prep.1997.0826.