Abstract
Background:
Biofilm formation is a major virulence factor in different bacteria. Biofilms allow bacteria to resist treatment with antibacterial agents. The biofilm formation on glass and steel surfaces, which are extremely useful surfaces in food industries and medical devices, has always had an important role in the distribution and transmission of infectious diseases.Objectives:
In this study, the effect of coating glass and steel surfaces by copper nanoparticles (CuNPs) in inhibiting the biofilm formation by Listeria monocytogenes and Pseudomonas aeruginosa was examined.Materials and Methods:
The minimal inhibitory concentrations (MICs) of synthesized CuNPs were measured against L. monocytogenes and P. aeruginosa by using the broth-dilution method. The cell-surface hydrophobicity of the selected bacteria was assessed using the bacterial adhesion to hydrocarbon (BATH) method. Also, the effect of the CuNP-coated surfaces on the biofilm formation of the selected bacteria was calculated via the surface assay.Results:
The MICs for the CuNPs according to the broth-dilution method were ≤ 16 mg/L for L. monocytogenes and ≤ 32 mg/L for P. aeruginosa. The hydrophobicity of P. aeruginosa and L. monocytogenes was calculated as 74% and 67%, respectively. The results for the surface assay showed a significant decrease in bacterial attachment and colonization on the CuNP-covered surfaces.Conclusions:
Our data demonstrated that the CuNPs inhibited bacterial growth and that the CuNP-coated surfaces decreased the microbial count and the microbial biofilm formation. Such CuNP-coated surfaces can be used in medical devices and food industries, although further studies in order to measure their level of toxicity would be necessary.Keywords
Biofilms Nanoparticles Listeria monocytogenes Pseudomonas aeruginosa
1. Background
Many bacteria have the potency to colonize and adhere to surfaces by producing a three-dimensional matrix of extracellular polymeric substances called the biofilm (1). Biofilms permit bacteria to persist in the environment and resist treatment with antibacterial agents, rendering their elimination very problematic (2-4). Bacteria, apart from the biofilm layer, can be a source of contamination and damage food quality, causing products to become the potential carriers of pathogens (5). Furthermore, it has been demonstrated that certain diseases such as periodontitis, cystic fibrosis, otitis media, native valve endocarditis, and chronic prostatitis all seem to be associated with biofilm-associated microorganisms (6). A wide range of indwelling medical devices used in the healthcare setting have been shown to harbor biofilms, resulting in calculable rates of device-associated infections (6).
Hydrophobic interactions are commonly the strongest of all long-range non-covalent forces, and the attachment of microorganisms to surfaces is often mediated by these types of interactions (7, 8). It has been found that the strains of Listeria monocytogenes with high hydrophobicity have a greater ability to attach to glass and steel surfaces (9). Also, studies suggest that there is a significant difference in biofilm formation between the different strains of a bacterium isolated from environmental, nosocomial, and food sources (10). Djordjevic et al. (11) noted that L. monocytogenes isolated from clinical samples have a greater ability to form biofilms than the other strains of this bacterium that are isolated from other origins (11). In addition, most antibiotic-resistant bacteria show the ability to form biofilms (12). It has also been mentioned that the intrinsic resistance of Pseudomonas aeruginosa to numerous antimicrobial agents is even more pronounced when this microorganism is found growing in a biofilm (2). Biofilms are thought to become recalcitrant to antimicrobial assault through a number of different mechanisms. In some instances, increased resistance may be caused by the poor diffusion of antibiotics through the biofilm polysaccharide matrix (13).
Recently, some studies have shown that metal nanoparticles (NPs) are able to prevent the formation of biofilms in microorganisms (14). These particles are considered to be a significant class of materials in the development of novel devices that can be used in various applications. Also, the antimicrobial activity of some metal NPs such as zinc, titanium, silver, copper, and aluminum in reducing the growth of different microorganisms have been studied (14, 15). The results of these studies suggest that heavy metal ions and metal oxide NPs, which have antibacterial characteristics, can prevent the formation of bacterial biofilms (14, 16).
2. Objectives
Since biofilm formation has a very important role in the development of infection and copper nanoparticles (CuNPs) have the ability to act as an antimicrobial agent, we decided to measure the effect of glass and steel surfaces coated with CuNPs to reduce the biofilm formation by L. monocytogenes and P. aeruginosa.
3. Materials and Methods
3.1. Synthesis of Copper Nanoparticles
In the current study, CuNPs (with a mean particle size of approximately 8 nm) were synthesized from pure copper metal wire (99.99% purity, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) using an inert gas condensation method described in a previous work (17). To prevent the NPs from agglomeration, the CuNPs were dispersed in pre-sterilized deionized water by ultrasonication (model 934, Yamato, Tokyo, Japan) after the particles were synthesized.
3.2. Bacterial Strains
Bacterial strains, namely Listeria monocytogenes PTCC 1298 and Pseudomonas aeruginosa PTCC 1310, were maintained, grown, subcultured in a trypticase soy broth (TSB) medium (Merck, Darmstadt, Germany), and stored at 4°C.
3.3. Minimum Inhibitory Concentration Assay of Copper Nanoparticles by Broth-Dilution Method
Minimum inhibitory concentrations (MICs) were calculated using the broth-dilution method for each bacterium. Serial dilutions of the CuNPs (512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, and 0.125 mg/L) were prepared in a Muller-Hinton broth (Merck, Darmstadt, Germany). Additionally, 1.5 × 108 CFU/mL of bacterial suspension was added to each tube and incubated at 37°C for 24 hours. The tubes were examined for turbidity, which indicated the growth of microorganisms. The lowest concentration of CuNPs that inhibits the growth of bacteria was designated as the MIC.
3.4. Hydrophobicity Assessment
Cell-surface hydrophobicity was measured using the bacterial adhesion to hydrocarbon (BATH) test. The BATH assay, developed by Rosenberg et al. (18), gives a cell hydrophobicity index (A% = percentage of adhesion). Cell suspensions were made based on a 0.5 McFarland standard, and 4 mL of cell suspension was transferred to individual test tubes, which contained 1 mL of octane. The test tubes were vortexed for 2 minutes and left to stand for 15 minutes to allow phase separation. The optical density (OD)640 of the aqueous phase was calculated, and the partitioning of the bacterial suspension was expressed as the percentage of the cells adsorbed by the hydrocarbon phase:
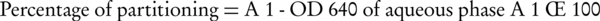
A1 was considered as primary OD640 of a cell suspension before adding octane hydrocarbon (19).
3.5. Surface Assay
Stainless steel and glass surfaces were selected since they are widely used in nosocomial environments as well as in industries related to medical devices and food processing. Some flat coupons of stainless steel and glass were covered with synthesized CuNPs, while some other coupons were kept uncovered as positive controls. In order to remove any grease from the surfaces, the coupons were primarily immersed in acetone and laid in a Decon FS200 sonication bath (Decon Ultrasonics Ltd., Hove, UK), including 5% Decon and 90% detergent. Furthermore, in order to remove all bacterial and extracellular polymeric substances, the coupons were sonicated for an hour. Then, the coupons were washed with tap water three times, and were finally autoclaved (121°C, 15 minutes, one bar).
The coupons were placed in 30 mL of sterile plastic universals (SterilinTM) so that both sides could be used for bacterial attachment. Regarding bacterial adherence, 1 μL of microbial suspension 106 CFU/mL, followed by 15 mL of the TSB medium, was added to the sterile plastic universals and stored in an incubator at 60 rpm at 28°C. Biofilm formation on the coupons was measured using the drop plate method at 8, 16, 24, 32, 40, and 48 hours (20). The coupons were washed with phosphate buffered saline (PBS) twice to remove non-adherent bacteria. Then, swabs were used and drawn to the surface of the bacterial biofilm on each coupon and vortexed in 10 mL of PBS. The bacterial suspension was made, and serial dilutions were subsequently prepared. Next, 10 μL of each concentration of L. monocytogenes was added to PALKAM agar (Merck, Darmstadt, Germany), and the same amount of P. aeruginosa suspension was added to nutrient agar (Merck, Darmstadt, Germany). This procedure was repeated three times for each dilution. After the development of colonies, the plates were removed; the dilution contained 3 - 30 colonies per 10 μL drop. Viable cell counts are expressed as colony forming units (CFU)/surface area.
4. Results
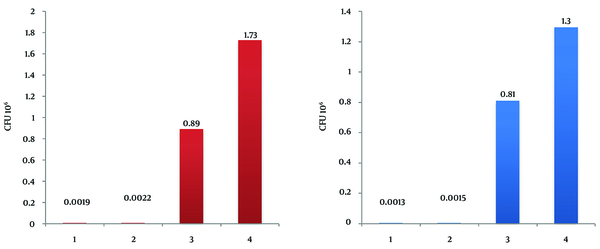
The results of the MICs for the CuNPs via the broth-dilution method were ≤ 32 mg/L for P. aeruginosa and ≤ 16 mg/L for L. monocytogenes. According to the test performed to analyze microbial linkage to octane via the BATH assay, the hydrophobicity of P. aeruginosa and L. monocytogenes was calculated as 74% and 67%, respectively. The antibacterial effect of CuNPs against biofilm formation on steel and glass surface was assessed by the quantity of viable bacterial cells. The numbers of L. monocytogenes on the glass and steel coupons and also on the CuNP-coated glass and steel coupons after 48 hours were counted as 8.1 × 105, 1.3 × 106, 1.3 × 103, and 1.5 × 103, while the corresponding numbers for P. aeruginosa were counted as 8.9 × 105, 1.73 × 106, 1.9 × 103, and 2.2 × 103 (Figure 1). Figures 2 and 3 show the biofilm formation of P. aeruginosa and L. monocytogenes on various surfaces continuously over a 48-hour period as assessed via the surface assay. The highest rate of attachment and colonization of microorganisms was witnessed in the first 8 hours, which demonstrated different patterns in the surfaces coated with CuNPs. In this study, we investigated higher colonization on steel surfaces in comparison to glass surfaces for both bacteria, and also higher tendency to attach to steel surfaces for P. aeruginosa in comparison to L. monocytogenes.
Biofilm Formation of Pseudomonas aeruginosa and Listeria monocytogenes
Pseudomonas aeruginosa Growth Curves
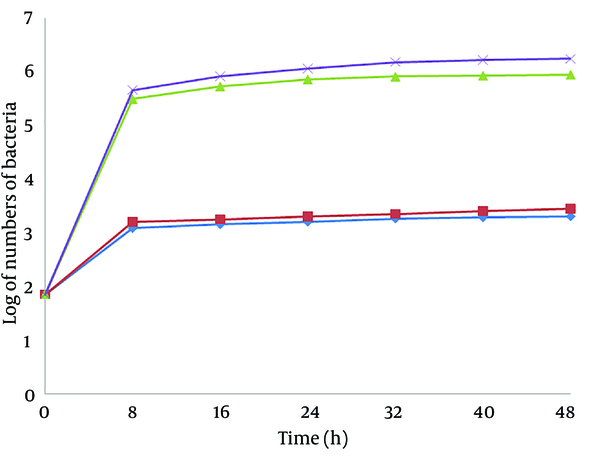
Listeria monocytogenes Growth Curves
5. Discussion
The present study found out that coating critical surfaces with CuNPs decreased the microbial count and microbial biofilm formation. Biofilm formation has been considered an important mechanism clearly harmful to hygiene industries (5, 21). The adherence of bacteria to different surfaces depends on such a large number of factors as hydrophobicity, cell-surface charge, electron acceptor, and donor properties (5, 9). Cerca et al. (7) explained that a high level of hydrophobicity is required for biofilm formation. Chiming in with our hydrophobicity test results, Lee and Yii (22) recorded a high level of hydrophobicity for P. aeruginosa and L. monocytogenes, which could account for their high ability to attach to surfaces. According to our results, P. aeruginosa and L. monocytogenes exhibited more tendencies to form biofilms on steel surfaces rather than glass surfaces, which can be explained as the high hydrophobicity of the bacteria and the specific characteristics of the bacterial strains used in this study. This is a finding of great significance because a large number of medical devices are made of steel. Also, given the differences between L. monocytogenes and P. aeruginosa in forming biofilms on similar glass and steel surfaces, the reason explaining this difference may be found within the different characteristics of both bacterial strains.
Moreover, the results obtained from this study confirm that CuNPs have inhibitory effects on selected bacterial species. The sensitivities of the two bacterial species to CuNPs were shown to be different. This is consistent with previous reports stating that the antimicrobial activity of CuNPs varies depending on microbial species (23, 24). Kim et al. (23) reported that different microbes vary in terms of antimicrobial susceptibility to NPs. Similarly, Ruparelia et al. (24), having studied the antimicrobial properties of silver NPs and CuNPs, stated that microbial sensitivity to NPs demonstrate differences depending on the microbial species. The results of the present research confirm the findings of the above-mentioned studies. The MICs for the CuNPs according to the broth-dilution method were ≤ 32 mg/L for P. aeruginosa and ≤ 16 mg/L for L. monocytogenes.
The antibacterial effect of NPs on bacteria depends on the available surface for interaction. When NPs have a larger available surface for interactions, their antibacterial effect tends to increase and they become more cytotoxic to microorganisms (25, 26). Smaller NPs can establish a wider contact surface and can, thus, be more active than large particles (27). In a study performed by Cousins et al. (26), the highest antimicrobial effect of silica NPs was observed in NPs with 7 and 14 nm. Our study confirms that CuNPs synthesized with a particle size of 8 nm have not only antibacterial but also antibiofilm effects. The noteworthy point in this study is a remarkable decrease in colonization on the surfaces coated with CuNPs (Figures 2 and 3). The highest rate of attachment and colonization of the microorganisms was recorded in the first 8 hours, demonstrating particularly different patterns in the CuNP-coated surfaces. This finding is concordant with the results of a study by Lellouche et al. (28), who explained the effect of coating surfaces with NPs on biofilm formation by Escherichia coli and Staphylococcus aureus. Therefore, the marked reduction in biofilm formation on CuNP-coated surfaces could be attributed to the inhibitory role of CuNPs on bacterial attachment. Our findings verify the antibiofilm behavior of surfaces coated with CuNPs and are, as such, in line with the results of a study conducted by Eshed et al. (29).
Considering the spread of industrial contamination, especially in food industries, and also the daily increasing spread of nosocomial infections, the importance of decreasing microbial contaminants on useful surfaces is obvious and this challenge would be more crucial given the fact that a wider range of bacteria show resistance to antimicrobial agents. CuNPs and CuNP-coated surfaces could harness the harmful effects of microbes. In this study, appropriate antimicrobial properties of CuNPs against L. monocytogenes and P. aeruginosa have been investigated. However, taking into account that copper and other metals may have toxic properties, using them as free NPs or as a cover on medical devices or materials for clinical use on living creatures needs further studies in toxicology.
Acknowledgements
References
-
1.
Borucki MK, Peppin JD, White D, Loge F, Call DR. Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol. 2003;69(12):7336-42. [PubMed ID: 14660383].
-
2.
De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, et al. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2001;45(6):1761-70. [PubMed ID: 11353623]. https://doi.org/10.1128/AAC.45.6.1761-1770.2001.
-
3.
Meyer B. Approaches to prevention, removal and killing of biofilms. Int Biodeterioration Biodegradation. 2003;51(4):249-53.
-
4.
Shafiei M, Abdi Ali A, Shahcheraghi F, Saboora A, Akbari Noghabi K. Eradication of Pseudomonas aeruginosa Biofilms Using the Combination of n-butanolic Cyclamen coum Extract and Ciprofloxacin. Jundishapur J Microbiol. 2014;7(2). ee14358. [PubMed ID: 25147668]. https://doi.org/10.5812/jjm.14358.
-
5.
Marques SC, Rezende J, Alves L, Silva BC, Alves E, Abreu LR, et al. Formation of biofilms by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitizers. Brazilian J Microbiol. 2007;38(3):538-43.
-
6.
Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881-90. [PubMed ID: 12194761]. https://doi.org/10.3201/eid0809.020063.
-
7.
Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res Microbiol. 2005;156(4):506-14. [PubMed ID: 15862449]. https://doi.org/10.1016/j.resmic.2005.01.007.
-
8.
Cunliffe D, Smart CA, Alexander C, Vulfson EN. Bacterial adhesion at synthetic surfaces. Appl Environ Microbiol. 1999;65(11):4995-5002. [PubMed ID: 10543814].
-
9.
Briandet R, Meylheuc T, Maher C, Bellon-Fontaine MN. Listeria monocytogenes Scott A: cell surface charge, hydrophobicity, and electron donor and acceptor characteristics under different environmental growth conditions. Appl Environ Microbiol. 1999;65(12):5328-33. [PubMed ID: 10583984].
-
10.
Harvey J, Keenan KP, Gilmour A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007;24(4):380-92. [PubMed ID: 17189764]. https://doi.org/10.1016/j.fm.2006.06.006.
-
11.
Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002;68(6):2950-8. [PubMed ID: 12039754].
-
12.
Potera C. Forging a link between biofilms and disease. Science. 1999;283(5409):1837. 1839. [PubMed ID: 10206887].
-
13.
Stewart PS. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 1996;40(11):2517-22. [PubMed ID: 8913456].
-
14.
Kanematsu H, Ikigai H, Yoshitake M. Evaluation of various metallic coatings on steel to mitigate biofilm formation. Int J Mol Sci. 2009;10(2):559-71. [PubMed ID: 19333421]. https://doi.org/10.3390/ijms10020559.
-
15.
Li Y, Leung P, Yao L, Song QW, Newton E. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62(1):58-63. [PubMed ID: 16099072]. https://doi.org/10.1016/j.jhin.2005.04.015.
-
16.
Sadiq IM, Chowdhury B, Chandrasekaran N, Mukherjee A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomedicine. 2009;5(3):282-6. [PubMed ID: 19523429]. https://doi.org/10.1016/j.nano.2009.01.002.
-
17.
Ghasemian E, Naghoni A, Tabaraie B, Tabaraie T. In vitro susceptibility of filamentous fungi to copper nanoparticles assessed by rapid XTT colorimetry and agar dilution method. J Mycol Med. 2012;22(4):322-8. [PubMed ID: 23518166]. https://doi.org/10.1016/j.mycmed.2012.09.006.
-
18.
Rosenberg M. Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol Lett. 1984;22(3):289-95.
-
19.
Mahdavi M, Jalali M, Kasra K. R. The Assessment of Biofilm Formation in Iranian Meat Processing Environments. Res J Microbiol. 2008;3(3):181-6.
-
20.
Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods. 2001;44(2):121-9.
-
21.
Mittal R, Khandwaha RK, Gupta V, Mittal PK, Harjai K. Phenotypic characters of urinary isolates of Pseudomonas aeruginosa & their association with mouse renal colonization. Indian J Med Res. 2006;123(1):67-72. [PubMed ID: 16567871].
-
22.
Lee KK, Yii KC. A comparison of three methods for assaying hydrophobicity of pathogenic vibrios. Letters Appl Microbiol. 1996;23(5):343-6.
-
23.
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95-101. [PubMed ID: 17379174]. https://doi.org/10.1016/j.nano.2006.12.001.
-
24.
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707-16. [PubMed ID: 18248860]. https://doi.org/10.1016/j.actbio.2007.11.006.
-
25.
Raffi M, Mehrwan S, Bhatti TM, Akhter JI, Hameed A, Yawar W, et al. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann Microbiol. 2010;60(1):75-80.
-
26.
Cousins BG, Allison HE, Doherty PJ, Edwards C, Garvey MJ, Martin DS, et al. Effects of a nanoparticulate silica substrate on cell attachment of Candida albicans. J Appl Microbiol. 2007;102(3):757-65. [PubMed ID: 17309625].
-
27.
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346-53. [PubMed ID: 20818017]. https://doi.org/10.1088/0957-4484/16/10/059.
-
28.
Lellouche J, Friedman A, Gedanken A, Banin E. Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int J Nanomedicine. 2012;7:5611-24. [PubMed ID: 23152681]. https://doi.org/10.2147/IJN.S37075.
-
29.
Eshed M, Lellouche J, Matalon S, Gedanken A, Banin E. Sonochemical coatings of ZnO and CuO nanoparticles inhibit Streptococcus mutans biofilm formation on teeth model. Langmuir. 2012;28(33):12288-95. [PubMed ID: 22830392]. https://doi.org/10.1021/la301432a.