Abstract
Background:
Extracellular phospholipase, proteinase, and coagulase are accounted as the major virulence factors in Candidaalbicans. Several reports showed that the incidence of resistance to fluconazole has risen during last two decades.Objectives:
The present study has investigated the extracellular enzymes of C. albicans and non-albicans species isolated from both patients with vaginitis and healthy women. In addition, susceptibility of the isolates was evaluated against fluconazole.Patients and Methods:
Vaginal samples were collected using sterile cotton swabs and inoculated on CHROMagar Candida. Routine morphological tests and ID 32C and API 20C AUX Kits were used to identify species. Phospholipase, proteinase, and coagulase activity were determined by standard methods. Susceptibility to fluconazole was also evaluated using ATB Fungus 3 Kits.Results:
The phospholipase activity was detected in 66.7% of the tested isolates recovered from patients with vaginitis. In the present study, phospholipase activity with higher Pz values (< 0.70) was more common in patients with vaginitis (28 of 66 isolates) whereas this rate in the normal individual was 13 of 42. Proteinase activity was detected in 74.2% and 61.9% of tested isolates recovered from patients and normal individuals, respectively. All tested isolates were negative for coagulase activity. In the present study, resistance to fluconazole was found in 34.8% of isolates. C. dubliniensis was the most common isolate (6 out of 11 isolates) that showed resistance to fluconazole.Conclusions:
Our results showed that C. albicans was the most frequently isolated from both patients with vaginitis and normal individual. In the present study, we could not find any correlation between extracellular activities and sources of isolates (patients and normal flora) and sensitivity or resistance to fluconazole.Keywords
Vulvovaginal Candidiasis Candida albicans Phospholipase Coagulase Fluconazole
1. Background
Candida vaginitis is a common opportunistic infection that affects women during childbearing period, as well as patients with diabetes and immunodeficiency (1, 2). Vulvovaginal candidiasis is considered as a common opportunistic mycosis in female genital system. This disease usually affects 75% of women in their life time (3). It is usually associated with considerable morbidity, healthcare cost, distress, pain, and sexual dysfunction (4). The predisposing factors such as immunodeficiency, diabetes mellitus, pregnancy, long-term treatment with corticosteroids, and oral contraceptives have an important role in the development of vulvovaginal candidiasis (2, 4).
Candida albicans is the most agent of Candida vaginitis followed by other species of Candida such as C. glabrata and C. tropicalis (4). Several reports have shown that the virulence of C. albicans is linked to pathogenicity factors such as adherence to epithelial cells, dimorphism (Y→M), cell surface composition, phenotypic switching, and thigmotropism (5-7). In addition, many virulence factors contribute to the pathogenicity of Candida, including production of extracellular enzymes (phospholipase, proteinase, coagulase, esterase and hemolytic activity), biofilm formation, and surface adherence (8). Extracellular phospholipases and proteases have an important role in damaging fungal cell membranes. Both enzymes play an active role in the invasion of hyphal tip to enter the host cells.
Several topical and oral antifungals such as fluconazole, ketoconazole, clotrimazole, butoconazole, and miconazole are used for the treatment of Candida vaginitis (9). Resistance to antifungals is considered as an important factor for the recurrence and or delay in the treatment, especially in immunocompromised patients. Fluconazole is a triazole antifungal used for the treatment of Candida vaginitis as a single dose (9). Several reports show that the incidence of the resistance to fluconazole has risen during last two decades (9-12).
2. Objectives
The present study investigated extracellular phospholipase, proteinase, and coagulase activities in different isolates of C. albicans and non-albicans species isolated from both patients with vaginitis and healthy women. In addition, susceptibility of the isolates was also evaluated against fluconazole.
3. Patients and Methods
3.1. Isolates and Preparation of Standard Suspension
Vaginal samples were collected using sterile cotton swabs and inoculated on CHROMagar Candida (CHROMagar Candida, France). Cultured media were aerobically incubated at 30 - 35°C for one week. Colonies producing a green coloration were presumptively identified as C. albicans. Furthermore, these species were confirmed by germ tube and chlamydoconidia formation. Other species were detected using CHROMagar Candida, ID 32C and API 20C AUX Kits (bioMerieux, France). A suspension of overnight cultures was prepared in phosphate-buffer solution (PBS) and adjusted with turbidity of 0.5 McFarland standards.
3.2. Phospholipase Activity
Phospholipase activity assays were performed according to Ge et al. (13) using Sabouraud dextrose agar (SDA, Merck, Germany) supplemented with egg yolk. Briefly, the test medium consisted of 6.5 g SDA, 5.84 g NaCl, 0.55 g CaCl2, and 2% egg yolk. Ten microliters of each standard suspension of strains was inoculated in triplicate and incubated at 25-27°C for 2 weeks (Figure 1). Colony diameter and the colony-plus precipitation zone were manually measured and the zone of phospholipase activity (Pz) was calculated. The Pz of 3 separate strains was measured and their average was calculated. The lower was the Pz value for each strain, the higher would be the phospholipase activity (14).
Phospholipase Precipitation Zone Around C. albicans
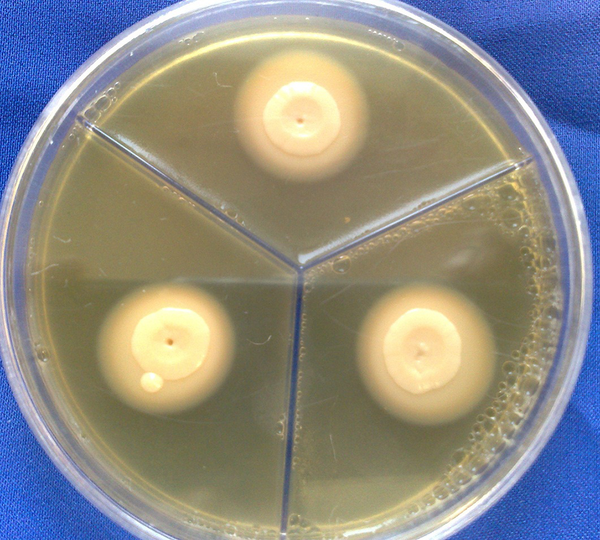
3.3. Proteinase Activity
Proteinase activity of isolates was evaluated using bovine serum albumin agar medium (BSA) (15). Medium contained 1% agar (Merck, Germany), 0.1% (wt/vol) KH2PO4, 0.5% (wt/vol) MgSO4, 1% (wt/vol) glucose, and 0.16% (wt/vol) BSA as the sole nitrogen source, pH 5.0. Ten microliters of each standard suspension of tested strains was placed onto the test medium and incubated at room temperature for 2 weeks. All experiments were carried out in triplicate. Then, the plates were fixed with trichloroacetic acid (20%) and stained with 1.25% Amido black (Cellogel, Italy). The clear zone around each colony was manually measured and the Pz value for each 3 separate strains was calculated as described previously and finally the average Pz calculated (Figure 2).
Proteinase Clear Zone Around C. albicans (Stained With Amido Black)
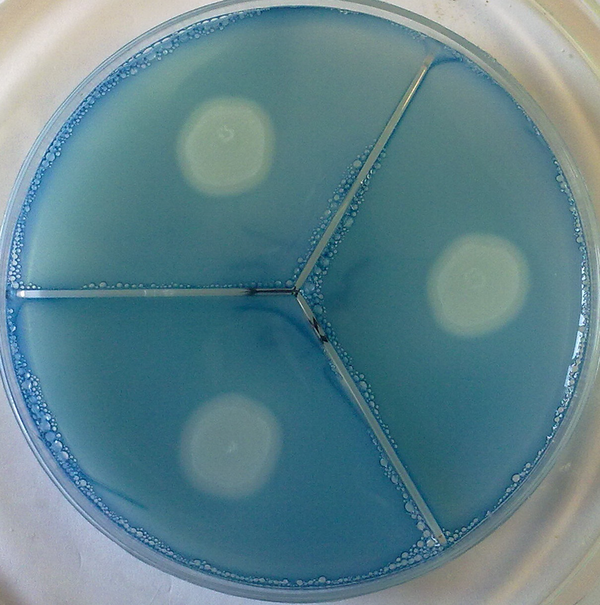
3.4. Coagulase Activity
The coagulase activity of the isolate was assessed using the rabbit plasma by a classical tube test. Zero point one milliliter of the culture of the isolate in Sabouraud glucose broth (Merck, Germany) was inoculated into a tube containing 0.5 mL of rabbit plasma and incubated at 30°C. A visible clot formation that could not be resuspended by gentle shaking at 2, 4, 6, and 24 hours was read as positive (5, 16). In addition, Staphylococcus aureus was used as positive control (5).
3.5. Susceptibility to Fluconazole
Susceptibility to fluconazole was determined using ATB Fungus 3 Kits (bioMerieux, France) (17).
4. Results
In the present study, several species of Candida were isolated from patients with vulvovaginal candidiasis and healthy individuals. C. albicans (47.2%) was the most common species among the isolates followed by C. glabrata (24.1%), C. dubliniensis (12%) and Candida species (5.6%). Table 1 shows the details of the isolates recovered from patients with Candida vaginitis and healthy individuals.
Frequency of Recovered Candida Species From Patients With Candida Vaginitis and Healthy Individualsa
| Candida Spp | Healthy Individuals | Patients With Candida Vaginitis |
|---|---|---|
| C. albicans | 19 (45.2) | 32 (48.5) |
| C. glabrata | 11 (26.2) | 15 (22.7) |
| C.dubliniensis | 2 (4.8) | 11 (16.7) |
| C. tropicalis | 1 (2.4) | 1 (1.5) |
| C. parapsilosis | 4 (9.5) | 0 (0.0) |
| C. guilliermondii | 0 (0.0) | 1 (1.5) |
| C. kefyr | 1 (2.4) | 3 (4.5) |
| C. humicola | 0 (0.0) | 1 (1.5) |
| Candida spp. | 4 (9.5) | 2 (3.1) |
4.1. Phospholipase Activity
In the present study, all recovered yeasts were examined for the production of extracellular phospholipase. The phospholipase activity was detected in 66.7% and 54.8% tested isolates recovered from patients with vaginitis and normal individuals, respectively (Table 2). Phospholipase activity with higher Pz values (< 0.70) was more common in vaginitis (42.5%) whereas this rate in the normal individual was 31%. Thirty-three point three percent of tested isolates from patients with vaginitis were without phospholipase activity in comparison to 45.2% in normal flora isolates. In our study, all 9 strains of C. parapsilosis isolated from healthy individuals were without phospholipase activity.
Phospholipase Production in 108 Isolates of Candida Species From Patients With Vaginitis and Healthy Individualsa
| Candida Spp | Healthy Individuals | Patients With Vaginitis | ||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| C. albicans | 2 (4.8) | 17 (40.5) | 5 (6.1) | 28 (42.4) |
| C. dubliniensis | 0 (0.0) | 2 (4.8) | 1 (1.5) | 10 (15.2) |
| C. glabrata | 9 (21.4) | 2 (4.8) | 12 (18.2) | 3 (4.5) |
| C. tropicalis | 1 (2.4) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| C. parapsilosis | 9 (21.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| C. guilliermondii | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| C. kefyr | 1 (2.4) | 0 (0.0) | 3 (4.5) | 0 (0.0) |
| C. humicola | 0 (0.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| Candida spp | 2 (4.8) | 2 (4.8) | 1 (1.5) | 1 (1.5) |
4.2. Proteinase Activity
In the present study, out of 66 recovered isolates from patients with vaginitis, 49 (74.2%) isolates were able to show proteinase activity. Whereas 26 (61.9%) strains that were isolated from normal individual had proteinase activity (Table 3). About 45.5% and 28.6% of isolates were found to be positive for the production of extracellular proteinase with Pz, 0.80 - 0.89 (++) isolated from vaginitis and normal individual, respectively.
Proteinase Production in 108 Isolates of Candida Species From Patients With Vaginitis and Healthy Individualsa
| Candida Spp | Healthy Individuals | Patients With Vaginitis | ||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| C. albicans | 7 (16.7) | 12 (28.6) | 8 (12.1) | 24 (36.4) |
| C. dubliniensis | 1 (2.4) | 1 (2.4) | 4 (6.1) | 7 (10.6) |
| C. glabrata | 7 (16.7) | 4 (9.5) | 3 (4.5) | 12 (8.2) |
| C. tropicalis | 0 (0.0) | 1 (2.4) | 1 (1.5) | 0 (0.0) |
| C. parapsilosis | 0 (0.0) | 4 (9.5) | 0 (0.0) | 0 (0.0) |
| C. guilliermondii | 0 (0.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| C. kefyr | 0 (0.0) | 1 (2.4) | 0 (0.0) | 3 (4.5) |
| C. humicola | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Candida spp | 1 (2.4) | 3 (7.1) | 0 (0.0) | 2 (3) |
4.3. Coagulase Activity
In our study, all tested isolates from patients with vulvovaginal candidiasis and healthy individuals were negative for coagulase activity.
4.4. Susceptibility to Fluconazole
We tested 66 isolates that were recovered from patients with Candida vulvovaginitis. In the present study, 34.8% of isolates were resistant to fluconazole whereas 53.1% and 12.1% were sensitive and dose dependent, respectively. C. dubliniensis was the most common isolate (6 of 11 isolates) that show resistance to fluconazole (Table 4).
| Candida Spp | Resistant | Dose Dependent | Sensitive |
|---|---|---|---|
| C. albicans | 13 (19.7) | 4 (6.1) | 15 (22.7) |
| C. dubliniensis | 6 (9.1) | 1 (1.5) | 4 (6.1) |
| C. glabrata | 3 (4.5) | 2 (3.1) | 10 (15.2) |
| C. tropicalis | 0 (0.0) | 1 (1.5) | 0 (0.0) |
| C. guilliermondii | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| C. kefyr | 0 (0.0) | 0 (0.0) | 3 (4.5) |
| C. humicola | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Candida spp | 1 (1.5) | 0 (0.0) | 1 (1.5) |
5. Discussion
In a study conducted by Tay et al., the positivity rates for protease and phospholipase activities were 93.7% and 73.3%, respectively (18). Other studies showed that the levels of extracellular enzymes of phospholipase and protease activities in clinical isolates of Candida were found to be higher than those in commensal isolates (14, 15). In a similar study, Ibrahim et al. found higher phospholipase activity in C. albicans isolates from the bloodstream in comparison to normal flora isolates of healthy individuals (19). Ombrella et al. have shown that 96.2% of vaginal isolates of Candida were positive for the production of phospholipase (15). On the other hand, in Vidotto et al. study, phospholipase and protease activity were found most frequently in oral cavity isolates (20). They believed that the productions of phospholipase and germ tube would accelerate the adhesion of C. albicans into membranes. Several reports have shown that the phospholipase activity of C. albicans was significantly higher than that of the non-C. albicans isolates (7, 8). However, Kantarcıoglu and Yucel could not find any differences in phospholipase activities between C. albicans and non-C. albicans strains (21).
Proteinase activity of recovered isolates from respiratory (93%) and blood (83%) samples were significantly higher than that of urine (77%), pus (65%), and stool isolates (60%) (21). Our study shows that the secretion of both extracellular enzymes of protease and phospholipase in Candida species recovered from the patients with vaginitis was higher than healthy individuals. Rodrigues et al. have shown that the most of isolates of C. albicans (88.5%) and C. tropicalis (82.6%) from blood, stool, urine, respiratory and genital secretions were able to produce coagulase enzyme at 24 hours (5). In another study that conducted by Yigit et al., 64.7% of C. albicans strains induced clot formation (16). Whereas our study shows that all pathogenic and normal strains of Candida species were negative for the production of coagulase enzyme. There is little information concerning Candida coagulase activity in the literature. In addition coagulase activity in Candida species may be related to pathogenicity, which is poorly investigated and requires more rigorous studies (22). During recent years, an increase in non-albicans species, particularly C. glabrata and C. tropicalis as the etiology of vulvovaginal candidiasis were spotted (4, 23).
Several studies were shown that both species are more resistant to fluconazole compared to C. albicans strains resistance (10, 24). Fluconazole resistance was also observed among C. glabrata and C. krusei isolated from vaginitis in Richter et al. study (25). Sojakova et al. have believed that 13% of vaginal isolates of Candida were resistant to fluconazole (26). On the other hand, in a study conducted by Mohanty et al. 30 isolates of Candida (C. glabrata, C. albicans, C. tropicalis and C. parapsilosis) originated from vaginitis were evaluated against fluconazole. They found that none of the tested isolates were resistant to fluconazole (3). Our study shows that 34.8% of vaginal isolates were resistant to fluconazole, including 13 of 32 C. albicans, and 10 of 34 non-albicans species. Some of the studies have shown that the level of extracellular enzymes in C. albicans isolates resistant to amphotericine B and fluconazole has increased (24, 27-29). However Kecli et al. have shown that there is no statistically correction between the phospholipase activity of 45 isolates of C. albicans and 5 isolates of non-albicans and resistant to fluconazole (29). Our study shows that there is not any significant correlation between the levels both extracellular enzymes and fluconazole sensitivity.
In conclusion, our results show that C. albicans was the most frequently isolated from both patients with vaginitis and normal individual. In the present study, we could not find any correlation between extracellular (phospholipase, proteinase, coagulase) activities and sources of isolates (patients and normal flora). However, the secretion of extracellular enzymes was higher in isolates from the patients compared to normal individuals.
Acknowledgements
References
-
1.
Rasti S, Asadi MA, Taghriri A, Behrashi M, Mousavie G. Vaginal candidiasis complications on pregnant women. Jundishapur J Microbiol. 2014;7(2). ee10078. [PubMed ID: 25147665]. https://doi.org/10.5812/jjm.10078.
-
2.
Mahmoudabadi AZ, Najafyan M, Moghimipour E, Alwanian M, Seifi Z. Lamisil versus clotrimazole in the treatment of vulvovaginal candidiasis. Iran J Microbiol. 2013;5(1):86-90. [PubMed ID: 23466900].
-
3.
Mohanty S, Xess I, Hasan F, Kapil A, Mittal S, Tolosa JE. Prevalence & susceptibility to fluconazole of Candida species causing vulvovaginitis. Indian J Med Res. 2007;126(3):216-9. [PubMed ID: 18037716].
-
4.
Mahmoudabadi AZ, Najafyan M, Alidadi M. Clinical study of Candida vaginitis in Ahvaz, Iran and susceptibility of agents to topical antifungal. Pak J Med Sci. 2010;26(3):607-10.
-
5.
Rodrigues AG, Pina-Vaz C, Costa-de-Oliveira S, Tavares C. Expression of plasma coagulase among pathogenic Candida species. J Clin Microbiol. 2003;41(12):5792-3. [PubMed ID: 14662985].
-
6.
Sun X, Lu H, Jiang Y, Cao Y. CaIPF19998 reduces drug susceptibility by enhancing the ability of biofilm formation and regulating redox homeostasis in Candida albicans. Curr Microbiol. 2013;67(3):322-6. [PubMed ID: 23620174]. https://doi.org/10.1007/s00284-013-0366-x.
-
7.
Jin Y, Samaranayake YH, Yip HK, Samaranayake LP. Characterization of switch phenotypes in Candida albicans biofilms. Mycopathologia. 2005;160(3):191-200. [PubMed ID: 16205967]. https://doi.org/10.1007/s11046-005-6331-x.
-
8.
Gokce G, Cerikcioglu N, Yagci A. Acid proteinase, phospholipase, and biofilm production of Candida species isolated from blood cultures. Mycopathologia. 2007;164(6):265-9. [PubMed ID: 17874282]. https://doi.org/10.1007/s11046-007-9053-4.
-
9.
Sekhavat L, Tabatabaii A, Tezerjani FZ. Oral fluconazole 150 mg single dose versus intra-vaginal clotrimazole treatment of acute vulvovaginal candidiasis. J Infect Public Health. 2011;4(4):195-9. [PubMed ID: 22000847]. https://doi.org/10.1016/j.jiph.2011.05.006.
-
10.
Pinto E, Ribeiro IC, Ferreira NJ, Fortes CE, Fonseca PA, Figueiral MH. Correlation between enzyme production, germ tube formation and susceptibility to fluconazole in Candida species isolated from patients with denture-related stomatitis and control individuals. J Oral Pathol Med. 2008;37(10):587-92. [PubMed ID: 18764856]. https://doi.org/10.1111/j.1600-0714.2008.00687.x.
-
11.
Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol. 2012;120(6):1407-14. [PubMed ID: 23168767].
-
12.
Danby CS, Boikov D, Rautemaa-Richardson R, Sobel JD. Effect of pH on in vitro susceptibility of Candida glabrata and Candida albicans to 11 antifungal agents and implications for clinical use. Antimicrob Agents Chemother. 2012;56(3):1403-6. [PubMed ID: 22232293]. https://doi.org/10.1128/AAC.05025-11.
-
13.
Ge YP, Lu GX, Shen YN, Liu WD. In vitro evaluation of phospholipase, proteinase, and esterase activities of Candida parapsilosis and Candida metapsilosis. Mycopathologia. 2011;172(6):429-38. [PubMed ID: 21698404]. https://doi.org/10.1007/s11046-011-9440-8.
-
14.
Mahmoudabadi AZ, Zarrin M, Miry S. Phospholipase activity of Candida albicans isolated from vagina and urine samples. Jundishapur J Microbiol. 2007;3(4):169-73.
-
15.
Ombrella AM, Racca L, Ramos L. [Protease and phospholipase activities of Candida albicans isolated from vaginal secretions with different pH values]. Rev Iberoam Micol. 2008;25(1):12-6. [PubMed ID: 18338921].
-
16.
Yigit N, Aktas E, Dagistan S, Ayyildiz A. Investigating biofilm production, coagulase and hemolytic activity in Candida species isolated from denture stomatitis patients. Eurasian J Med. 2011;43(1):27-32. [PubMed ID: 25610156]. https://doi.org/10.5152/eajm.2011.06.
-
17.
Instruction for ATBTM Fungus bS. France; 2011.
-
18.
Tay ST, Abidin IA, Hassan H, Ng KP. Proteinase, phospholipase, biofilm forming abilities and antifungal susceptibilities of Malaysian Candida isolates from blood cultures. Med Mycol. 2011;49(5):556-60. [PubMed ID: 21254967]. https://doi.org/10.3109/13693786.2010.551424.
-
19.
Ibrahim AS, Mirbod F, Filler SG, Banno Y, Cole GT, Kitajima Y, et al. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63(5):1993-8. [PubMed ID: 7729913].
-
20.
Vidotto V, Yumi Koga-Ito C, Milano R, Fianchino B, Ponton J. Correlation between germ tube production, phospholipase activity and serotype distribution in Candida albicans. Rev Iberoam Micol. 1999;16(4):208-10. [PubMed ID: 18473549].
-
21.
Kantarcioglu AS, Yucel A. Phospholipase and protease activities in clinical Candida isolates with reference to the sources of strains. Mycoses. 2002;45(5-6):160-5. [PubMed ID: 12100532].
-
22.
Yigit N, Aktas AE, Ayyildiz A. Detection of coagulase activity in pathogenic Candida species. J Int Med Res. 2008;36(6):1378-82. [PubMed ID: 19094449].
-
23.
Mahmoudi Rad M, Zafarghandi S, Abbasabadi B, Tavallaee M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):199-203. [PubMed ID: 21194828]. https://doi.org/10.1016/j.ejogrb.2010.11.022.
-
24.
da Costa KR, Ferreira JC, Komesu MC, Candido RC. Candida albicans and Candida tropicalis in oral candidosis: quantitative analysis, exoenzyme activity, and antifungal drug sensitivity. Mycopathologia. 2009;167(2):73-9. [PubMed ID: 18787978]. https://doi.org/10.1007/s11046-008-9154-8.
-
25.
Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005;43(5):2155-62. [PubMed ID: 15872235]. https://doi.org/10.1128/JCM.43.5.2155-2162.2005.
-
26.
Sojakova M, Liptajova D, Borovsky M, Subik J. Fluconazole and itraconazole susceptibility of vaginal yeast isolates from Slovakia. Mycopathologia. 2004;157(2):163-9. [PubMed ID: 15119851].
-
27.
Kumar R, Shukla PK. Amphotericin B resistance leads to enhanced proteinase and phospholipase activity and reduced germ tube formation in Candida albicans. Fungal Biol. 2010;114(2-3):189-97. [PubMed ID: 20943129]. https://doi.org/10.1016/j.funbio.2009.12.003.
-
28.
Lyon JP, de Resende MA. Correlation between adhesion, enzyme production, and susceptibility to fluconazole in Candida albicans obtained from denture wearers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):632-8. [PubMed ID: 17052640]. https://doi.org/10.1016/j.tripleo.2005.12.015.
-
29.
Kecli K, Budak F, Lden SG, Tamer N, Willke A. An investigation on the antifungal susceptibility and phospholipase activity of Candida species. infection Mag. 2003;17(3):321-4.
