Abstract
Background:
Methicillin-Resistant Staphylococcus aureus (MRSA) are bacteria responsible for several difficult-to-treat infections in humans. These strains have developed, through the process of natural selection. Infections by MRSA are more difficult to treat with standard types of antibiotics and thus more dangerous to human health.Objectives:
The aim of this study was to evaluate the bactericidal and antibiotic synergistic effect of silver nanoparticles (Ag-NPs) against MRSA.Materials and Methods:
Methicillin-Resistant Staphylococcus aureus strains were isolated from clinical samples and identified, and their susceptibility was tested using the MicroScan® WalkAway-96® SI System. minimum inhibitory concentration (MIC) was determined by a microdilution method. Time kill assay was performed by exposing the MRSA isolates to different concentrations of Ag-NPs and monitoring bacterial growth, by measuring optical density at 600 nm. Tissue culture plate was used for determination of the efficacy of Ag-NPs and their combination with antibiotics in the elimination of formed biofilm.Results:
The MIC value of Ag-NPs against MRSA was 100 μg/mL. Methicillin-Resistant Staphylococcus aureus cells were treated with 50, 100 and 200 µg/mL of Ag-NPs and inhibited bacterial growth so that after four hours, almost all treated MRSA cells were dead. All combinations showed effectiveness against MRSA. It was observed that MRSA did not show inhibition zones with ampicillin alone.Conclusions:
Silver Nanoparticles have high therapeutic activity against MRSA, thus can be suggested as an alternative or adjuvant with antibiotics for MRSA treatment. Further studies are required to understand the synergistic effect of Ag-NPs combinations and to assess the safety and efficacy of new antibiotic-Ag-NPs combinations.Keywords
Ag-NPs Nanosilver Antibiotics Methicillin-Resistant Staphylococcus aureus
1. Background
Methicillin-Resistant Staphylococcus aureus (MRSA) is a pathogen that has received great medical interest, as it has not been eliminated from healthcare institutions or the environment (1). A bacteremia caused by S. aureus leads to between 25% and 63% mortality; the medical importance of MRSA is due to the high rate of mortality and morbidity associated with its infections and it is considered the main cause of nosocomial infections worldwide (2). The world health organization has reported an MRSA prevalence of about 70% to 80% of all the S. aureus isolates in some Asian countries (3). A total of 92 (26.3%) S. aureus isolates were obtained, of which 33 (35.9%) were MRSA and 27 (29.3%) were Multi-Drug Resistant Staphylococcus aureus (MDRSA) in Iran (4). Currently there is a critical need to find alternative antimicrobials, especially those that could be used for treatment of MRSA infections (5). Amongst several antimicrobial compounds that have been investigated, Ag-NPs showed promising antimicrobial properties, which could be used for treatment MRSA and other drug-resistant infections.
Silver Nanoparticles (Ag-NPs) (less than 100 nm) have shown novel antimicrobial activity to a wide range of microorganisms due to their high surface area to volume ratio and their unique chemical and physical properties when compared to the properties of their bulk form (6). These Ag-NPs could be applied safely in therapy when effective concentrations against many types of organisms have been determined. Recently, we demonstrated that Ag-NPs show antifungal activities, including apoptotic cell death. In particular, we showed that hydroxyl radicals play an important role in apoptosis (7). In addition, many researches have investigated the synergistic effect of Ag-NPs when combined with other compounds: a combination of amoxicillin and Ag-NPs showed better antibacterial properties against Escherichia coli than when they were applied alone (8). The production of biofilm is related to antibiotic resistance problems (9). Bacteria strongly adhere to surfaces using a biofilm matrix, 3D, gel-like, highly hydrated and locally charged environment. Adhesion of these bacteria to other organs may contribute to the pathogenesis of infection (10). Biofilms are often associated with dental plaques (11, 12), endocarditis (13), lung infection (14) and infection through medical devices (15, 16).
2. Objectives
In the present study, we investigated the antimicrobial and anti-biofilm activities of Ag-NPs alone and in combination with conventional antibiotics against pathogenic bacteria.
3. Materials and Methods
3.1. Bacterial Isolates
Eighty S. aureus isolates were obtained from clinical specimens at the microbiology laboratory of the Department of Clinical Analysis at the Gynecology and Children Hospital, Hafr Elbatin, KSA. Mid-stream urine, suction tip, pus and blood specimens were collected aseptically for bacteriological examination. Handling, transporting and storing of collected samples were made at refrigeration temperature. All samples were inoculated on blood agar, incubated at 37°C overnight, and colonies were processed.
3.2. Antimbiogram and Methicillin-Resistant Staphylococcus aureus Identification
The MicroScan® WalkAway-96® SI System (Siemens Healthcare Diagnostics Inc, USA) was used for laboratory identification, while antimicrobial susceptibility testing and MRSA detection was performed using Neg/BP/Combo 30-B1017-306E® combination panels (Siemens Healthcare Diagnostics Inc, USA). All procedures were performed according to the manufacturer’s instructions. The well-diffusion method was used to evaluate synergistic effects of Ag-NPs with antibiotics.
3.3. Antimicrobial Activity of Silver Nanoparticles
Silver Nanoparticles were obtained from Sigma (St. Louis, MO, USA). Minimum inhibitory concentration (MIC) was defined as the lowest concentration of Ag-NPs preparation that prevented bacterial growth. The MIC was determined using the micro-dilution method; using LB broth (Sigma, St. Louis, MO, USA) MRSA concentration was adjusted to the McFarland standard. The MRSA isolates were exposed to serial twofold dilutions of Ag-NPs, and MRSA viability was measured after 24 hours of incubation. The MRSA cell viability was measured using a colony-forming capacity assay on nutrient agar (17). All the assays were done with a negative and a positive control.
3.4. Time- Kill Assay
To examine the growth curves of MRSA, isolates were incubated in Mueller-Hinton broth (Sigma, St. Louis, MO, USA) with different concentrations of Ag-NPs (0, 50, 100, and 200 μg/ml), and the MRSA cells concentration was adjusted to McFarland standard and cultures were incubated in a shaking incubator at 37°C for 24 hours. Growth curves of MRSA cultures were constructed by measuring of the optical density (OD) at 600 nm at one-hour intervals for seven hours.
3.5. Synergistic Effect of Silver Nanoparticles With Antibiotics
The well diffusion method was used to evaluate the synergistic of Ag-NPs with antibiotics, based on the Clinical and Laboratory Science Institute (CLSI) standards (18). Briefly, Mueller-Hinton agar (Sigma, St. Louis, MO, USA) plates were inoculated with the turbidity-adjusted MRSA suspension and an antibiotic (HiMedia Chemicals Pvt. Ltd., Mumbai, India), and were incubated at 37°C for 24 hours. Next, the inhibition zone (ZOI) diameter of Ag-NPs, antibiotic and Ag-NPs + antibiotic combination were measured, by subtracting the well diameter from the total inhibition zone diameter. Synergistic effect was calculated by the following equation:
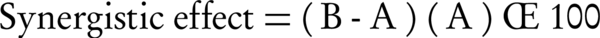
Where A is ZOI for the antibiotic and B is ZOI for the antibiotic + Ag-NPs (19).
3.6. Anti-Biofilm Activity of Nanosilver by the Tissue Culture Plate (TCP) Method
To investigate the anti-biofilm activity of Ag-NPs alone and in combination with antibiotics, a TCP assay was performed (20). Individual wells of sterile, polystyrene, 96-well, flat-bottomed TCPs were filled with 190 µL of the bacterial suspension at 105 CFU. After 18 hours of overnight culture, Ag-NPs alone or in combination with antibiotic was added with the final concentration being the MIC. The Ag-NPs were replaced by deionized water in the control well. The TCPs were incubated for eight hours at 37°C, and the wells were then washed four times with 0.2 ml of PBS, heat-fixed at 60°C for one hour and stained with 0.4% crystal violet for 15 minutes. The stain was rinsed off, resolubilized with DMSO (Sigma, St. Louis, MO, USA) and absorbance was measured at 570 nm (Spectramax M2e, Molecular Devices, CA, USA). The control was considered to represent 100% of biofilm formation. The percentage of biofilm inhibition was calculated using the following equation (21):
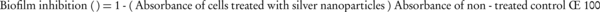
3.7. Statistical Analysis
All experiments were performed in triplicates, and means were determined. Results were expressed as percentages.
4. Results
4.1. Bacterial Strains
Of the eighty S. aureus isolates, 30 were MRSA, Methicillin-Resistant Staphylococcus aureus.
4.2. Antimicrobial Activity of Silver Nanoparticles
The MRSA isolates were exposed to twofold Ag-NPs serial dilutions for 24 hours. Silver Nanoparticles affected MRSA cellular viability in a dose-dependent manner. Cell viability assay was used to determine the bactericidal effect of different concentrations of silver nanoparticles on MRSA. Bacterial cell viability was measured using a colony-forming capacity assay in nutrient agar. The MRSA isolates were inhibited at concentrations over 100 µg/mL at 105 CFU where no visible bacterial growth was observed in the agar plates Figure 1.
Colony-Forming Capacity Assay
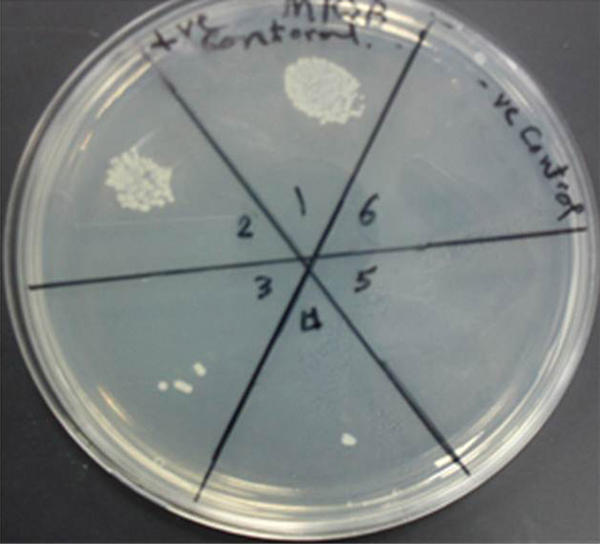
4.3. Time-Kill Assay
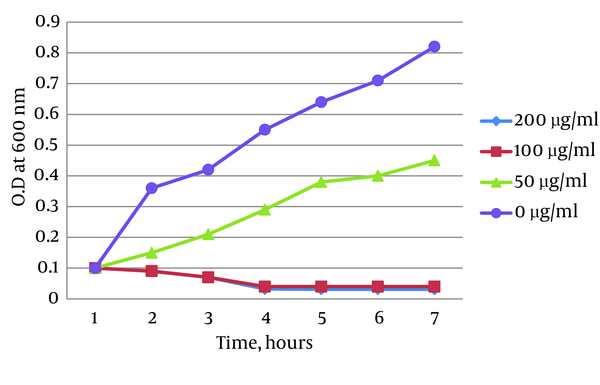
Time-kill assay showed that Ag-NPs could inhibit the growth of MRSA. The growth curves of MRSA treated with 50, 100 and 200 µg/mL Ag-NPs were affected in a concentration-dependent manner and after four hours, almost all treated MRSA were dead. There was a small difference between growth of MRSA treated with 50 µg/mL Ag-NPs and the control group Figure 2.
Methicillin-Resistant Staphylococcus aureus Growth Curve With Different Concentrations of Silver Nanoparticles
4.4. Synergistic Effect of Nanosilver With Antibiotics
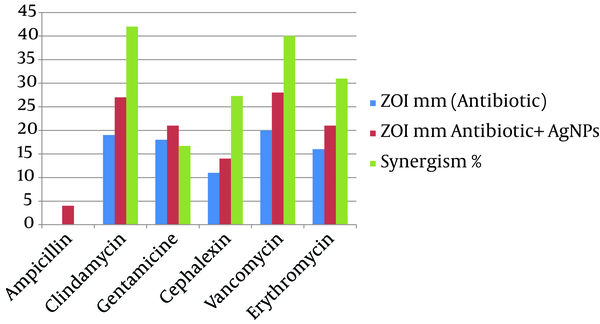
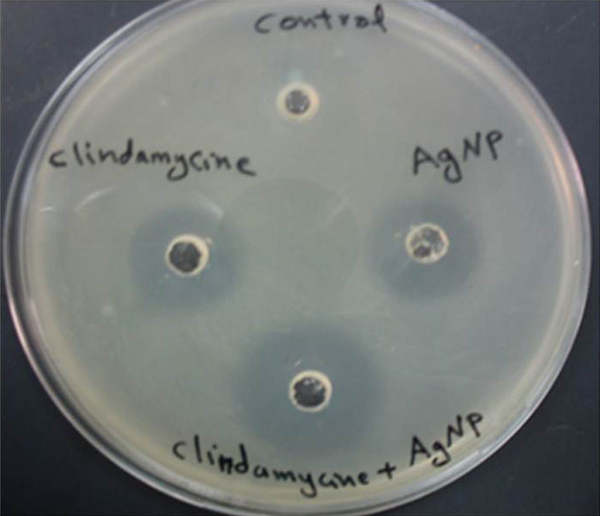
The synergistic effects of Ag-NPs were investigated with six conventional antibiotics against MRSA, using the well diffusion method and the effects were evaluated by determination of synergism percentage. The results of the combination assay are presented in Figure 3. All of the combinations showed effectiveness against the MRSA. It was observed that MRSA did not show an inhibition zone with ampicillin alone and the highest synergistic effect was observed in the presence of clindamycine Figure 4.
Average Inhibition Zone (mm) In the Presence of an Antibiotic, and Antibiotic Plus Silver Nanoparticles
Synergistic Effect of Nano-Silver (Ag-Nps) With Clindamycin
4.5. Determination of Biofilm Formation by the Tissue Culture Plate (TCP) Method
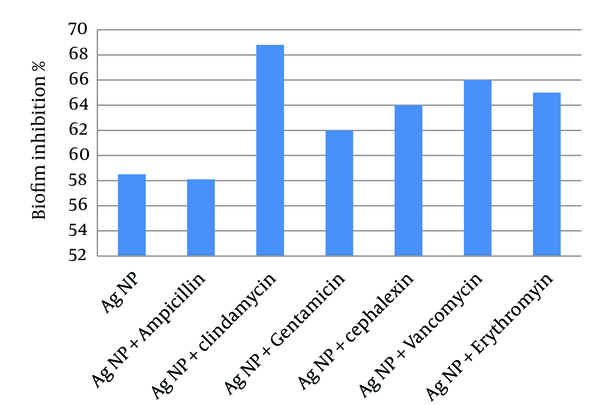
To examine the anti-biofilm activity of Ag-NPs and their combination with antibiotics, MRSA were grown to form biofilms and then exposed to Ag-NPs alone or in combination with the antibiotics (Figure 5). The results showed that Ag-NPs had an inhibitory activity on biofilm formation greater than 55%. Combinations of Ag-NPs and antibiotics, showed a greater inhibitory activity than Ag-NPs alone, yet ampicillin did not show any increase in activity and anti-biofilm activity was due to Ag-NPs.
Inhibition of Methicillin-Resistant Staphylococcus aureus Biofilm Formation Determined Using the Tissue Culture Plate Method
5. Discussion
The increased resistance to antibiotics is alarming as treating infections of resistance strains could be problematic. Methicillin-Resistant Staphylococcus aureus are important pathogens with increasing rates of multi-antibiotic resistance due to both intrinsic and acquired mechanisms. This study aimed to examine the antibacterial activity of Ag-NPs against MRSA, using different in vitro assays. To achieve this goal, we challenged thirty clinical isolates classified as MRSA with different concentrations of Ag-NPs and described the effect on MRSA viability, growth rate and biofilm formation. Also we studied the synergistic effect of Ag-NPs with antibiotics to find new formulas of more effective antibiotics to overcome the antibiotic resistance problem.
The results showed that the MRSA isolates were fully susceptible to the antibacterial effect of Ag-NPs over the MIC concentration. This proves the results obtained by other authors, which showed colloidal silver is a powerful antibiotic against a wide range of microorganisms at a very low concentration without having any harmful effect on body tissues (22, 23). Small Ag-NPs with large surface areas provide an effective antimicrobial agent even at very low concentrations. Silver Nanoparticles (diameter of 5 - 100 nm) enhances the antibacterial activity of various antibiotics (24, 25). Our results showed a 100% influence by nanosilver at the MIC of 100 µg/mL. These findings indicate that the antibacterial activity of 50 μg/mL of Ag-NPs could slightly inhibit bacterial growth yet not enough to outpace the speed of reproduction of the bacterial cells. Numerous researches have been performed studying the effect of Ag-NPs on pathogenic microorganisms. Our results showed a synergistic effect between nanosilver and antibiotics (Figures 3 and 4).
These results are somewhat similar to the results obtained by Fayaz et al. (26) and Rai et al. (27) on Pseudomonas aeruginosa. Interestingly, amongst the selected antibiotics, clindamycin in combination with Ag-NPs showed the highest activity against MRSA (42%), followed by vancomycin (40%) and erythromycin (31%). The synergistic effect of Ag-NPs and clindamycin was greater when compared to other antibiotics, which may be due to both, the inhibiting protein synthesis action of the clindamycin and the DNA binding action of the Ag-NPs; similar results were obtained by Shahverdi et al. (28). The probable mechanism involved in the synergistic effect of antibiotics with Ag-NPs could be the formation of complexes between the antibiotics and Ag-NPs; though the bonding of the antibiotic’s active and functional groups like hydroxyl and amino groups to the large surface area of Ag-NPs by chelating (26, 29). Silver Nanoparticles antibacterial mechanism works by inhibiting oxygen metabolism, which finally kills the microbes in a very short time (28).
The isolates of MRSA showed zero sensitivity to ampicillin; this effect did not show synergism when combined with Ag-NPs. Generally, the results of the antibiotic-Ag-NPs combination studies reported that Ag-NPs could enhance the antimicrobial activity of some antibiotics. However, many mechanisms of action were suggested for Ag-NPs antimicrobial activity such synergistic effect may not be completely clear (30). Also, the strong antimicrobial effect of Ag-NPs is due to attachment to cell receptors and inhibition of important metabolic enzymes resulting in disruption of microbial cell reproduction and respiration. Our results revealed that the killing rate was not concentration-dependent (31). Long-term cultivation enables bacterial cells to adhere to animal tissues and surfaces (32). This, in turn, results in the biofilm formation, a multilayered community of sessile bacterial cells. Bacteria growing in a biofilm are very problematic in hospitals and healthcare facilities due to their high antimicrobial resistance than planktonic cells. According to the National Institutes of Health, 80% of chronic infections are due to biofilms (33).
In this study combinations of Ag-NPs and antibiotics also led to an increase in inhibitory effect on biofilm formation with different degrees, yet, generally showed a greater inhibitory activity than Ag-NPs or antibiotic alone. These are encouraging results, as it may be possible to achieve an effective anti-biofilm effect at lower antibiotic concentrations. Therefore, Ag-NPs and their combination with antibiotics could be used as an anti-biofilm agent against bacterial biofilms. From our study findings, it could be concluded that Ag-NPs have high antibacterial activity against MRSA, and could be an alternative to antibiotics for treatment of drug-resistant microorganisms specially MRSA infection, or enhancement of conventional antibiotics activity against MRSA and other pathogenic microorganisms. Further studies are required to understand the synergistic effect of nanosilver combination, and assess the safety and efficacy of new antibiotic- Ag-NPs combinations.
Acknowledgements
References
-
1.
Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12 Suppl):S122-9. [PubMed ID: 15577930]. https://doi.org/10.1038/nm1145.
-
2.
Lim SM, Webb SA. Nosocomial bacterial infections in Intensive Care Units. I: Organisms and mechanisms of antibiotic resistance. Anaesthesia. 2005;60(9):887-902. [PubMed ID: 16115251]. https://doi.org/10.1111/j.1365-2044.2005.04220.x.
-
3.
Monitoring of Antimicrobial Resistance. Tamil Nadu; 2003.
-
4.
Erami M, Soltani B, Taghavi Ardakani A, Moravveji A, Haji Rezaei M, Soltani S, et al. Nasal Carriage and Resistance Pattern of Multidrug Resistant Staphylococcus aureus Among Healthy Children in Kashan, Iran. Iran Red Crescent Med J. 2014;16(10). ee21346. https://doi.org/10.5812/ircmj.21346.
-
5.
Motevasel M, Okhovat MA, Zomorodian K, Farshad S. A Study of the Effect of Zataria multiflora Extract on Methicillin Resistant Staphylococcus aureus. Jundishapur J Microbiol. 2013;6(5). https://doi.org/10.5812/jjm.5453.
-
6.
Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3(6):1-10. https://doi.org/10.1186/1477-3155-3-6.
-
7.
Hwang IS, Lee J, Hwang JH, Kim KJ, Lee DG. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012;279(7):1327-38. [PubMed ID: 22324978]. https://doi.org/10.1111/j.1742-4658.2012.08527.x.
-
8.
Martínez-Castañón GA, Niño-Martínez N, Martínez-Gutierrez F, Martínez-Mendoza JR, Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanoparticle Res. 2008;10(8):1343-8. https://doi.org/10.1007/s11051-008-9428-6.
-
9.
Lynch SV, Dixon L, Benoit MR, Brodie EL, Keyhan M, Hu P, et al. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob Agents Chemother. 2007;51(10):3650-8. [PubMed ID: 17664315]. https://doi.org/10.1128/AAC.00601-07.
-
10.
Archer GL. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998;26(5):1179-81. [PubMed ID: 9597249].
-
11.
Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13(6):508-12. [PubMed ID: 17944664]. https://doi.org/10.1111/j.1601-0825.2007.01410a.x.
-
12.
Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88(11):982-90. [PubMed ID: 19828884]. https://doi.org/10.1177/0022034509346811.
-
13.
Palmer J. Bacterial biofilms in chronic rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2006;196:35-9. [PubMed ID: 17040016].
-
14.
Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 2005;13(8):389-97. [PubMed ID: 15951179]. https://doi.org/10.1016/j.tim.2005.05.011.
-
15.
Wagner VE, Iglewski BH. P. aeruginosa Biofilms in CF Infection. Clin Rev Allergy Immunol. 2008;35(3):124-34. [PubMed ID: 18509765]. https://doi.org/10.1007/s12016-008-8079-9.
-
16.
Khardori N, Yassien M. Biofilms in device-related infections. J Ind Microbiol. 1995;15(3):141-7. [PubMed ID: 8519469].
-
17.
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797-810. [PubMed ID: 17803904]. https://doi.org/10.1016/j.cell.2007.06.049.
-
18.
Clinical and Laboratory Standards Institute. Informational Supplement. Wayne, Pennsylvania: CLSI Document M100-S16; 2006.
-
19.
Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 2014;9(1):373. [PubMed ID: 25136281]. https://doi.org/10.1186/1556-276X-9-373.
-
20.
Antunes AL, Trentin DS, Bonfanti JW, Pinto CC, Perez LR, Macedo AJ, et al. Application of a feasible method for determination of biofilm antimicrobial susceptibility in staphylococci. APMIS. 2010;118(11):873-7. [PubMed ID: 20955460]. https://doi.org/10.1111/j.1600-0463.2010.02681.x.
-
21.
Wei GX, Campagna AN, Bobek LA. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother. 2006;57(6):1100-9. [PubMed ID: 16595638]. https://doi.org/10.1093/jac/dkl120.
-
22.
Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68(7):4018-23. [PubMed ID: 10858217].
-
23.
Singh M, Singh S, Prasad S, Gambhir IS. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest J Nanomater Biostructures. 2008;3(3):115-22.
-
24.
Orji JO. Antibacterial efficacy of colloidal silver alone and in combination with other antibiotics on isolates from wound Infections. Sci Res Essays. 2007;2(8):338-41.
-
25.
Kim SH, Lee HS, Ryu DS, Choi SJ, Lee DS. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J Microbiol Biotechnol. 2011;39(1):77-85.
-
26.
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine. 2010;6(1):103-9. [PubMed ID: 19447203]. https://doi.org/10.1016/j.nano.2009.04.006.
-
27.
Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 2012;112(5):841-52. [PubMed ID: 22324439]. https://doi.org/10.1111/j.1365-2672.2012.05253.x.
-
28.
Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3(2):168-71. [PubMed ID: 17468052]. https://doi.org/10.1016/j.nano.2007.02.001.
-
29.
Oni AA, Nwaorgu OGB, Bakare RA, Ogunkunle MO, Toki RA. The Discharging Ears In Adults In Ibadan, Nigeria Causative Agents And Antimicrobial Sensitivity Pattern. Afr J Clin Exp Microbiol. 2002;3(1). https://doi.org/10.4314/ajcem.v3i1.7341.
-
30.
Marshall CR, Killoh GB. The Bactericidal Action of Collosols of Silver and Mercury. Br Med J. 1915;1(2820):102-4. [PubMed ID: 20767445].
-
31.
Costerton JW, Irvin RT, Cheng KJ. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299-324. [PubMed ID: 7027902]. https://doi.org/10.1146/annurev.mi.35.100181.001503.
-
32.
Costerton JW, Lappin-Scott HM. Introduction to microbial biofilms. Microbial Biofilms. 1995:1-11.
-
33.
Monroe D. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 2007;5(11). eee307. [PubMed ID: 18001153]. https://doi.org/10.1371/journal.pbio.0050307.