Abstract
Background:
Developing algal industries in saline-alkali areas is necessary. However, suitable strains and optimal production conditions must be studied before widespread commercial use.Objectives:
The effects of light, temperature, KNO3, and CO(NH2)2 on beta-carotene and biomass accumulation were compared and evaluated in order to provide scientific guidance for commercial algal production in northeastern Thailand.Materials and Methods:
An orthogonal design was used for evaluating optimal conditions for the algal production of three candidate Dunaliella salina strains (KU XI, KU 10 and KU 31) which were isolated from saline soils and cultured in the column photobioreactor.Results:
The optimal light and temperature for algae growth were 135.3 μmol m-2 s-1 and 22°C, while the conditions of 245.6 μmol m-2 s-1 and 22°C induced the highest level of beta-carotene production (117.99 mg L-1). The optimal concentrations of KNO3, CO(NH2)2, and NaHCO3 for algae growth were 0.5 g L-1, 0.36 g L-1, and 1.5 g L-1, respectively, while 0, 0.12 g L-1 and 1.5 g L-1 were best suited for beta-carotene accumulation. The highest beta-carotene rate per cell appeared with the highest light intensity (12.21 pg) and lowest temperature (12.47 pg), and the lowest total beta-carotene content appeared at the lowest temperature (15°C). There was not a significant difference in biomass accumulation among the three Dunaliella strains; however, the beta-carotene accumulation of KU XI was higher than that of the other two strains.Conclusions:
Light and temperature were both relevant factors that contributed to the growth and beta-carotene accumulation of the three D. salina strains, and NaHCO3 had significantly positive effects on growth. The degree of impact of the different factors on cell growth was temperature > NaHCO3 > light intensity > KNO3 > CO (NH2)2 > strains; the impact on beta-carotene accumulation was temperature > light intensity > KNO3 > CO (NH2)2 > strains > NaHCO3Keywords
1. Background
Beta-carotene is a naturally occurring orange-colored pigment synthesized by high plants and microorganisms (1-3). It is an important pigment in the process of photosynthesis in that it provides photo-oxidation protection, and functions as a medium in the general process (3). Beta-carotene is also an important source of nutrition since it can be converted into vitamin A (4). Moreover, it also has physiological effects, since it can function as a peroxyl radical scavenger, an immune response stimulant and a gap-junction communication enhancer (4, 5). Recent studies have shown that Dunaliella salina, which contains high levels of beta-carotene, is safe and can be a potential food supplement (6). Nowadays, beta-carotene is extensively used as a colorant, a food additive, an antioxidant, an anti-cancer agent, a preventative supplement against heart disease, and for cosmetic purposes (7).
Although a large portion of commercially available beta-carotene is chemically synthesized, there has been considerable interest in the production of natural beta-carotene from living organisms (8, 9). Dunaliella salina is a unicellular green alga belonging to the Chlorophyceae family. It is known to accumulate carotenoids under various conditions of stress, such as high salinity, high light intensity, and low growth temperature (10, 11). Dunaliella salina can tolerate a variety of environmental stresses, and is able to accumulate a beta-carotene percentage of up to 10% of its dry weight (12). Hence, the most common method for the commercial production of natural beta-carotene is the massive cultivation of D. salina (4). Present research on D. salina has made significant process in terms of the screening and selection of algae species or strains, medium optimization, cultivation, and general processing for production (13, 14). However, these experimental results, although fruitful, have yet to be directly applied to actual commercial production on a broad scale (14). The northeastern area of Thailand covers more than one-third of the country’s 16.9 million ha, including 9.25 million ha of agricultural land. There are about 2.8 million ha of saline soils, 17% of the total area of northeastern Thailand. Developing algal industries in saline-alkali areas is an important economic development strategy for this particular area (15).
Considering the future of commercial algal cultivation, we have focused on D. salina strains that were isolated directly from the saline soils in this area. Research has confirmed that different species or strains require different stress conditions to obtain high beta-carotene content (14); however, little is known about the exact impacts of different environmental or nutritional factors on the D. salina strains isolated from the saline soils. Some studies have shown that growth of D. salina was optimal when the nitrate concentration was ranged from 0.5 to 1 g L-1, however some researchers have found that growth in low nitrate concentrations (0.05 - 0.1 g L-1) was also substantial (4, 16, 17). Therefore, evaluating the effects of various factors on the algal production of the three D. salina strains is necessary for more conclusive results. Moreover, considering the high costs of commercial cultivation, CO (NH2)2 was also studied as a potential (more economical) replacement for KNO3 in this study.
2. Objectives
The difference in the growth and beta-carotene accumulation of D. salina strains under different stress conditions were studied in order to determine the optimal conditions and the best D. salina strain for commercial algal production. The impacts of different environmental factors on growth and beta-carotene accumulation were evaluated based on the results of an orthogonal experimental design in order to provide scientific guidance for the further optimization of production in northeastern Thailand.
3. Materials and Methods
3.1. Algae Strains and Cultivation Conditions
Dunaliella salina strains were obtained from the Bioscience Laboratory of the Botany Department at Kasetsart University. Three D. salina strains, namely KU XI, KU 10 and KU 31, were isolated from 60 saline soil samples in Thailand. KU XI was used as a control strain in contrast to the other two strains (18). Dunaliella salina strains were cultured in a column photobioreactor with a working volume of 250 ml (15). The culture medium was Modified Johnsons Medium (15). The initial pH and cell density were controlled at 7.5 and approx. 50 × 104/mL. The salinity (NaCl concentration) was 2 M (117 g NaCl L-1).
3.2. The Effects of Environmental Factors on Dunaliella salina
In order to study the effects of environmental factors on the growth and pigment accumulation of the three D. salina strains, a L9 (34) orthogonal form was designed (Table 1). Light intensity and temperature were determined based on the conditions in an actual algal cultivation plant located at Nakhon Ratchasima, Thailand. KNO3 was 0.5 g L-1; NaHCO3 was 0.5 g L-1, while the other components of the Modified Johnsons Medium were maintained at the original concentration. The light source was daylight fluorescence lamps with 12 hours light/12 hours dark.
Orthogonal Design of Light, Temperature (Temp.), and Strains
| No. | Light, μmolm -2 s-1 | Temp°C | Strains |
|---|---|---|---|
| Tr1 | 68.5 | 15 | KU 10 |
| Tr2 | 68.5 | 22 | KU Ⅺ |
| Tr3 | 68.5 | 30 | KU 31 |
| Tr4 | 135.3 | 15 | KU Ⅺ |
| Tr5 | 135.3 | 22 | KU 31 |
| Tr6 | 135.3 | 30 | KU 10 |
| Tr7 | 245.6 | 15 | KU 31 |
| Tr8 | 245.6 | 22 | KU 10 |
| Tr9 | 245.6 | 30 | KU Ⅺ |
3.3. The effects of Nutritional Factors on D. salina
For studying the effects of KNO3, CO (NH2)2 and NaHCO3 on the growth and pigment accumulation in the three D. salina strains, an L9 (34) orthogonal forms was designed (Table 2). The light intensity was 68.5 μmol m-2 s-1 and the light/dark circle was 12 hours/12 hours. The temperature was 22°C.
Orthogonal Design of Nutrition and Strains
| No. | Strains | KNO3, gL-1 | CO(NH2)2, gL-1 | NaHCO3, gL-1 |
|---|---|---|---|---|
| Tr1 | KU 10 | 0 | 0.12 | 0.5 |
| Tr2 | KU 10 | 0.5 | 0.24 | 1 |
| Tr3 | KU 10 | 1 | 0.36 | 1.5 |
| Tr4 | KU Ⅺ | 0 | 0.24 | 1.5 |
| Tr5 | KU Ⅺ | 0.5 | 0.36 | 0.5 |
| Tr6 | KU Ⅺ | 1 | 0.12 | 1 |
| Tr7 | KU 31 | 0 | 0.36 | 1 |
| Tr8 | KU 31 | 0.5 | 0.12 | 1.5 |
| Tr9 | KU 31 | 1 | 0.24 | 0.5 |
3.4. Cell Density Determination and Pigment Extraction
Algal densities were checked daily with a haematocytometer under a microscope, and the pH level was measured with a BT-10 pH meter manufactured by Becthai Bangkok Equipment and Chemical Co., LTD. For pigment extraction, centrifuged algae samples were dissolved into cold methanol with 0.1% (w/v) butylated hydroxytoluene (BHT) added, sonicated for 10 minutes. Finally, acetone (90%) was used to extract pigments from these processed samples. All procedures were conducted under dim laboratory light to prevent pigment photo-oxidation (7). The extracted solutions were examined by a HP8453 ultraviolet spectrophotometer manufactured by the Hewlett-Packard Company, USA. The chlorophyll content was calculated by using the following equation (15, 18): Chl = (8.02 × OD645 + 20.21 × OD663) × Vt/V0 Where Chl was chlorophyll content (mgL-1); OD645and OD663 were optical densities of pigment extraction solution; Vt was ultimate extraction solution volume (mL); and V0 was algae sample volume (mL).
The beta-carotene extraction solution was analyzed by a Waters e2695 HPLC with a Waters XTerra RP18 Column 5µm, manufactured by the Waters Company, USA. In the mobile phase, solvent A was ethyl acetate and solvent B was acetonitrile and water (9:1, v/v). The flow rate was 1 ml min-1. The solvent programming was 0 – 16 minutes, 0 – 60% solvent A; 16 – 30 minutes, 60% solvent A; 30 - 35 minutes, 100% solvent A (7, 19). Finally, the beta-carotene content was converted into dry biomass weight (mg g-1 dry biomass wt).
3.5. Statistical Analysis
The data was processed using Microsoft Excel 2010 software using the ANOVA method and t-Test. All treatments were evaluated four times.
3.6. Ethics Statement
The three D. salina strains, KU XI, KU 10, and KU 31, belonged to the Bioscience Laboratory of the Botany Department in the Faculty of Science at Kasetsart University. This lab has authorized all authors of this paper to use the three strains for scientific research work only.
4. Results
4.1. The Effect of Light, Temperature, and Strain on the pH Value of the Culture Medium
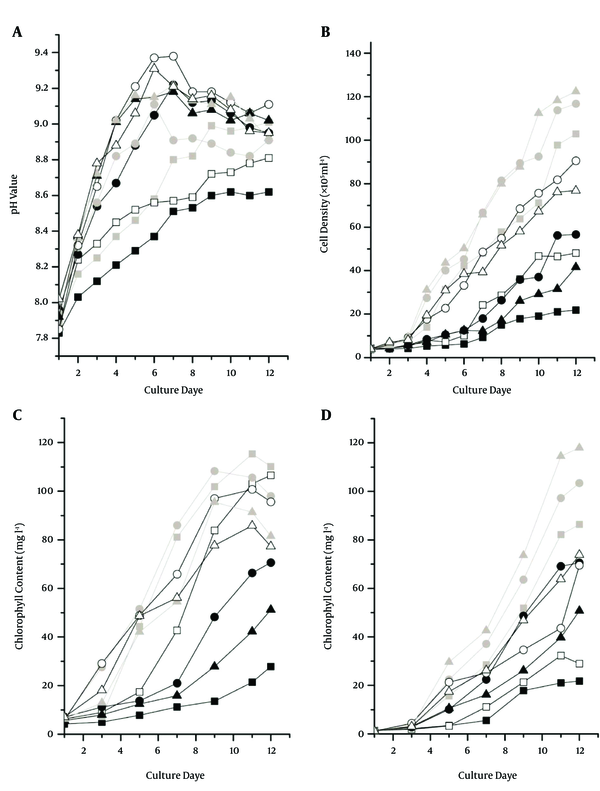
The pH value of cultures at 22°C and 30°C increased quickly and then gradually decreased after six or seven days. Tr6 and Tr1 showed the highest and lowest pH value, respectively. The pH value of cultures at 15°C (Tr1, Tr4, and Tr7) increased slowly and remained lower than the other two temperatures (Figure 1 A). Low temperature and light intensity restrained algae growth, resulting in a lower pH (20-22). The pH value was correlated with algae growth; cultures in the early stage of development all had lower pH values (Figure 1).
Effects of Environmental Factors on Strains
4.2. The Effects of Light and Temperature on the Growth of the Three Strains
The highest and lowest cell density and growth rate were presented in Tr8 and Tr1, respectively. The cell densities of cultures Tr8 and Tr9 under higher light intensity (245.6 μmol m-2 s-1) were significantly higher than the cultures Tr3 and Tr1 under lower light intensity. The other treatments did not show significantly positive or negative effects on the increase of cell density (Figure 1B). Moreover, the cell density of Tr2 (the lowest light intensity but the optimal temperature) was significantly higher than Tr7 (the highest light intensity but the lowest temperature). These results show that low light intensity could restrain growth; however, light intensity was not the only factor that was determined to have affected growth.
The growth of cultures at 22℃ (Tr8, Tr5, and Tr2) was better than with other treatments. Although the final cell density of Tr6 (30°C) was not significantly different when compared with cultures at 22°C, its growth rate in the early stage was lower than that of cultures at 22°C (Figure 1B). These results have shown that 22°C in this study was the most suitable temperature for algae growth. The orthogonal analysis of cell growth showed that light intensity and temperature both had significant effects on the growth of the three D. salina strains, and the impacts of temperature were stronger than the impacts of light intensity. The growth among strains was not significantly different (Table 3). This indicated that temperature could have more of an effect on algae growth; the optimal temperature and light intensity in this study were 22°C and 135.3 µmol m-2 s-1.
Orthogonal Analysis of Cell Growth Effected by Light, Temperature and Strains
4.3. The Effects of Light and Temperature on Pigment Accumulation in the Three Strains
Chlorophyll accumulation in six treatments of cultures at 22°C and 30°C increased quickly after three days of cultivation and declined after eight days. The final chlorophyll content was not significantly different, but the highest chlorophyll content appeared in Tr2 and Tr3, which were exposed to the lowest light intensity (68.5 μmol m-2 s-1). It can be concluded that low light intensity was most appropriate for chlorophyll accumulation (Figure 1C).
Chlorophyll accumulation in three treatments of cultures at 15°C was always lower than the other treatments in two temperature groups. The chlorophyll content of the three strains always displayed an ascendant trend in the cultures at 15°C, although their final content was lower than the content of the other treatments (Figure 1C). This could be explained by the fact that the three strains in the early cultivation stage needed different amounts of time to adapt to the low temperature, which resulted in low nutrient consumption and a low growth rate. Therefore, the nutrition, specifically nitrogen resource, still remained in the culture medium in the late stage which could continue maintain the algae growth (20, 21, 23). The beta-carotene accumulations of all cultures quickly increased after five days of cultivation. It could be explained that fast increases in biomass accelerated nutrient consumption which promoted beta-carotene accumulation (24). The highest beta-carotene content (117.99 mgL-1) appeared in Tr8, followed by Tr5. The final beta-carotene content of strain KU XI (Tr2, Tr4 and Tr9) was higher than that of the other two strains (Figure 1D).
Orthogonal analysis showed that temperature and light intensity both had significant effects on beta-carotene accumulation, and the impact of temperature was stronger than that of light intensity (Table 4). This indicated that temperature could be the main factor contributing to beta-carotene accumulation. The accumulation of beta-carotene in the three strains was not significantly different, although KU XI produced higher levels of beta-carotene than the other two strains (Table 4). The most suitable light intensity and temperature rates for beta-carotene accumulation of algae in this study were 135.3 μmol m-2 s-1 and 22°C. Beta-carotene content under the light intensity of 245.6 µmol m-2 s-1 and 135.3 µmol m-2 s-1 was not significantly different (Table 4). Moreover, 135.3 µmol m-2 s-1 and 22°C were also suitable for algae growth (Figure 1B). Hence, these rates could be used in actual algal production; controlling light intensity and temperature could simultaneously obtain maximum biomass and high levels of beta-carotene.
Orthogonal Analysis of Beta-carotene Accumulation Effected by Light, Temperature and Strains
4.4. The Effects of Nutrition and Strains on the pH Value of the Culture Medium
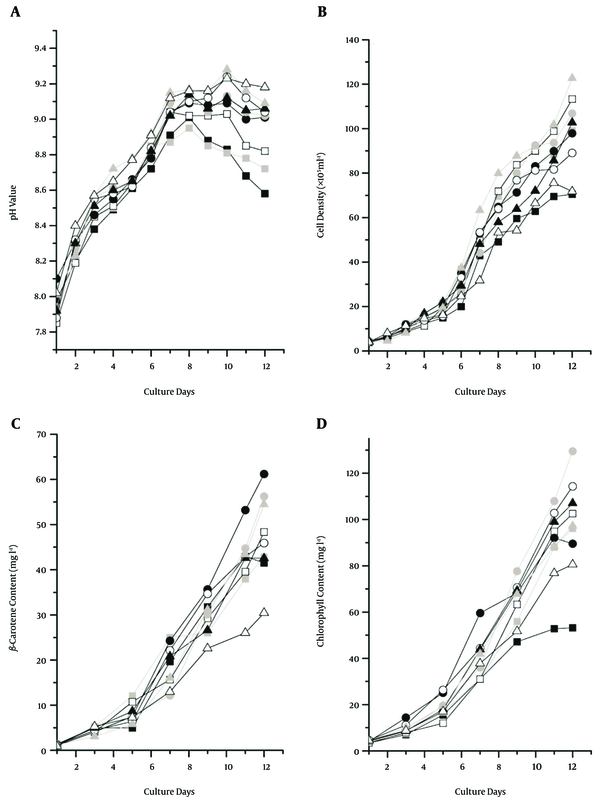
The pH values of all cultures all increased linearly before the seventh day, and then showed an irregular variation. This trend was different from previous light-temperature experiment results (Figure 1A and Figure 2A). Cultures with a high concentration of NaHCO3 (Tr3, Tr4 and Tr8) exhibited the highest pH value. Furthermore, their growth was better than that of the other cultures (Figure 2). Accordingly, cultures with a low concentration of NaHCO3 (Tr9, Tr5, and Tr1) showed the lowest pH value (Figure 2A). These results indicate that NaHCO3 could significantly affect the pH value of the culture medium.
Effects of Nutritional Factors on Strains
4.5. The effects of Nutrition on the Growth of the Three Strains
The growth of all cultures increased slowly before the fifth day, and their cell densities were not significantly different. The highest final cell densities were presented in Tr8, Tr3, and Tr4, and lowest final cell densities appeared in Tr9 and Tr1 (Figure 2B). However, their nitrogen concentrations were extremely different (Table 2). This indicates that nitrogen does not significantly affect growth; orthogonal analysis also confirmed this result (Table 5). Furthermore, the growth of the cultures with a high concentration of NaHCO3 (1.5 gL-1) was higher than those with a low concentration (0.5 gL-1) (Figure 2B). Moreover, the growth of cultures in different nitrogen resource was not significant different, although growth of cultures in mixed nitrogen resources was higher than that in CO (NH2)2 only (Figure 2B and Table 5).
Orthogonal Analysis of Cell Growth Affected by Nutrition and Strains
An orthogonal analysis of the growth affected by nutrition and strains showed that the effect of NaHCO3 on algae growth was positive. However, the growth among the strains was not significantly different. This result was consistent with previous experimental results (Tables 3 and 5). The optimal nutrition conditions for algae growth in this study were 0.5 g KNO3 L-1, 0. 36 g CO(NH2)2 L-1 and 1.5 g NaHCO3 L-1 (Table 5).
4.6. Effects of Nutrition on Pigment Accumulation in the Three Strains
Beta-carotene accumulation in all cultures rapidly increased after the fifth day. The highest beta-carotene content appeared in Tr4 (41.49 mgL-1) and Tr1 (61.19 mgL-1) with nitrogen resources of CO(NH2)2, but the concentration of NaHCO3 was inconsistent (Figure 2C and Table 2). KU XI accumulated higher beta-carotene levels than the other two strains (Figure 2C). Orthogonal analysis showed that the extremum value of beta-carotene accumulation among strains was higher than the growth value (Tables 5 and 6). Compared with previous experiment results, this showed that the beta-carotene accumulation ability of KU XI was stronger than that of the other two strains. Low concentrations of nitrogen were most suitable for beta-carotene accumulation (Table 6), but the effects of NaHCO3 on beta-carotene accumulation were weaker than the effects on algae growth (Tables 5 and 6). The results of this study have shown that the optimal conditions for beta-carotene accumulation were 0.12 g CO(NH2)2 L-1 and 1.5 g NaHCO3 L-1 (Table 6).
Orthogonal Analysis of Beta-carotene Accumulation Affected by Nutrition and Strains
Chlorophyll accumulations of all cultures quickly increased after 5 days cultivation, and their accumulation rate and content were all higher than the corresponding levels of β-carotene (Figure 2D). Higher chlorophyll content appeared in Tr5, Tr6, and Tr7, and their total nitrogen concentrations were higher than in the other treatments. Lower chlorophyll content appeared in Tr1 with the lowest nitrogen concentration (0.12 g L-1). Results indicated that high nitrogen concentration was most suitable for chlorophyll accumulation.
5. Discussion
Temperature and light intensity are both important environmental factors here, and studies found that temperature plays a more important role in growth rate than pH level (22). In this study, a temperature of 22°C had significant effects on D. salina growth. However, some reports indicated that 30°C was the most optimal growth temperature (7, 25, 26). The results of this study were consistent with some reports that suggested that 22 or 25°C were most suitable for D. salina growth (15, 27, 28). The three strains in this study were selected and maintained at 20°C. Borowitzka et al. (29) have suggested that D. salina grew better than the other organisms because of its strong adaptability in stressed environments (4). Hence, long-periods of environmental adaptability could have changed some of the living habits of D. salina. Results of this study also showed that 135.3 μmol m-2 s-1 was the optimal light intensity for algae growth, and that lower or higher light intensity could restrain algae growth.
The ionization balance of the medium was determined as follows: HCO3- → OH-+CO2. All D. salina are strictly photoautotrophs and able to uptake CO2; the HCO3- can be converted to CO2 by extracellular carbonic anhydrase (15). Due to rapid cell division and growth during the cultivation period, algae consumed amounts of CO2 that promoted high concentrations of OH-, resulting in an increased pH value. However, the pH value declined with continued cultivation over several. This result could be attributed to the large amounts of beta-carotene accumulation and some acidic chemicals secreted by algae cells, as well as the neutralized parts of the alkaloid chemicals (30).
Higher light intensity applied to each single cell with lower density in the early cultivation stage lead to higher levels of beta-carotene accumulation (27). High light intensity can hurt cellular development and restrain algae growth; therefore, the photosynthetic mechanism of D. salina was activated in order to produce larger amounts of beta-carotene, one of most important protective pigments located between the thylakoids and stored in neutral lipid droplets, effectively capable of filtering the abundant harmful light (27). Hence, high light intensity was suitable for beta-carotene biosynthesis but was disadvantageous to algae growth (5, 31). The orthogonal analysis of pigment accumulation and growth showed that D. salina growth depended on certain concentrations of nitrogen and carbon. However, current research on optimal nitrogen concentrations for algae growth had variable results, e.g. suitable nitrogen concentration (N) was N = 10 mM (4, 16) or N = 5 mM (17), both of which are consistent with the results of this study. However, some reports have claimed that growth under lower nitrogen concentrations (N = 0.75 or N = 1 mM) also performed well (14, 16).
CO(NH2)2 was more effective in promoting algae growth and KNO3 was most suitable for beta-carotene accumulation. Carbon was also important for algae growth and beta-carotene accumulation (16). Because the alga constantly utilizes HCO3- and uptakes CO2, the concentration of HCO3- decreased and the CO32- concentration increased, which further induced the precipitation of other ions (Ca2+ and Mg2+) (14, 32). Hence, high concentrations of NaHCO3 (1.5 g L-1) in this study were optimal for algae growth. However, the effects of NaHCO3 on beta-carotene accumulation were not significant. It could be explained that the algae cells can synthetize a series of chemicals under the suitable concentrations of nitrogen and carbon in order to maintain normal metabolic development. In the absence of nitrogen, the amounts of carbon and hydrogen will participate in non-nitrogen-induced pigment synthesis and initiate beta-carotene accumulation. Hence, the effects of nitrogen on beta-carotene accumulation were stronger than those of NaHCO3 (5, 14, 31).
According to results of this study, under the temperature and light intensity of 22°C and 245.6 μmol m-2 s-1, biomass and beta-carotene were simultaneously able to reach a high yield. Moreover, according to algal productivity in this study (0.11 g beta-carotene L-1 and 1.25 g biomass L-1 respectively), yields of one production period could be upwards of 29.7 kg pure β-carotene and 337.5 kg D. salina powder within 1000 m2 of a working area (valid working volume is 30 m × 30 m × 0.3 m). This yield is still lower than the intensive cultivation model. However, nutritional or environmental factors could be further optimized based on the results of this study in order to obtain higher yields. The commercial algal production plant was in Nakhon Ratchasima, Thailand, where most of the area is saline-alkali, with annual temperatures ranging between 22 - 33°C and 2800 hours of sunlight, suitable for algae cultivation. The most suitable commercial algal cultivation models in Nakhon Ratchasima, Thailand were small-scale outdoor race-way pond cultivation and intensive indoor cultivation. The significance of this study is that the results could be used in future intensive commercial cultivation projects and provide scientific guidance for future extensive commercial cultivation. The experimental strains were new and isolated from saline soil in the domestic area. Furthermore, experimental factors surrounding the design and cultivation environments were simulated to be as similar to actual conditions as possible. Hence, this research may have expansive value and application.
Acknowledgements
References
-
1.
Li Z, Ma X, Li A, Zhang C. A novel potential source of beta-carotene: Eustigmatos cf. polyphem (Eustigmatophyceae) and pilot beta-carotene production in bubble column and flat panel photobioreactors. Bioresour Technol. 2012;117:257-63. [PubMed ID: 22617035]. https://doi.org/10.1016/j.biortech.2012.04.069.
-
2.
McNeil B, Archer D, Giavasis I, Harvey L. Microbial production of food ingredients, enzymes and nutraceuticals. Cambridge: Elsevier; 2013.
-
3.
Cuttriss AJ, Cazzonelli CI, Wurtzel ET, Pogson BJ. Carotenoids. United States of America: Elsevier; 2011. p. 1-36.
-
4.
Borowitzka M. Microalgae as sources of pharmaceuticals and other biologically active compounds. J Appl Phycol. 1995;7(1):3-15.
-
5.
Ye ZW, Jiang JG, Wu GH. Biosynthesis and regulation of carotenoids in Dunaliella: progresses and prospects. Biotechnol Adv. 2008;26(4):352-60. [PubMed ID: 18486405]. https://doi.org/10.1016/j.biotechadv.2008.03.004.
-
6.
Lam MK, Lee KT. Scale-Up and Commercialization of Algal Cultivation and Biofuel Production. Amsterdam: Elsevier; 2014. p. 261-86. https://doi.org/10.1016/B978-0-444-59558-4.00012-7.
-
7.
Prieto A, Pedro Canavate J, Garcia-Gonzalez M. Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J Biotechnol. 2011;151(2):180-5. [PubMed ID: 21111012]. https://doi.org/10.1016/j.jbiotec.2010.11.011.
-
8.
Garcia-Gonzalez M, Moreno J, Manzano JC, Florencio FJ, Guerrero MG. Production of Dunaliella salina biomass rich in 9-cis-beta-carotene and lutein in a closed tubular photobioreactor. J Biotechnol. 2005;115(1):81-90. [PubMed ID: 15607227]. https://doi.org/10.1016/j.jbiotec.2004.07.010.
-
9.
Kleinegris DM, Janssen M, Brandenburg WA, Wijffels RH. Continuous production of carotenoids from Dunaliella salina. Enzyme Microb Technol. 2011;48(3):253-9. [PubMed ID: 22112908]. https://doi.org/10.1016/j.enzmictec.2010.11.005.
-
10.
Chidambara Murthy KN, Vanitha A, Rajesha J, Mahadeva Swamy M, Sowmya PR, Ravishankar GA. In vivo antioxidant activity of carotenoids from Dunaliella salina--a green microalga. Life Sci. 2005;76(12):1381-90. [PubMed ID: 15670617]. https://doi.org/10.1016/j.lfs.2004.10.015.
-
11.
Borowitzka M,A. Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol. 1999;70(1-3):313-21. https://doi.org/10.1016/S0168-1656(99)00083-8.
-
12.
Ben-Amotz A. New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol. 1995;7(1):65-8. https://doi.org/10.1007/BF00003552.
-
13.
Lamers PP, Janssen M, De Vos RC, Bino RJ, Wijffels RH. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008;26(11):631-8. [PubMed ID: 18752860]. https://doi.org/10.1016/j.tibtech.2008.07.002.
-
14.
Mou CL, Hao XH, Liu X, Chen XW, Chen DF. Cell factory of carotenoids-progress in Dunaliella cultivation and its research. Adv Mar Sci. 2010;28(4):554-62.
-
15.
Sathasivam R, Juntawong N. Modified medium for enhanced growth of Dunaliella strains. Int J Curr Sci. 2013;5:67-73.
-
16.
Borowitzka LJ. Development of western biotechnology's algal β-carotene plant. Bioresour Technol. 1991;38(2):251-2. https://doi.org/10.1016/0960-8524(91)90164-F.
-
17.
Ben-Amotz A, Katz A, Avron M. Accumulation of β-carotene in halotolerant algae: purification and characterization of β-carotene rich globules from Dunaliella bardawil. J Phycol. 1982;18(4):529-37. https://doi.org/10.1111/j.0022-3646.1982.00529.x.
-
18.
Sathasivam R, Kermanee P, Roytrakul S, Juntawong N. Isolation and molecular identification of β-carotene producing strains of Dunaliella salina and Dunaliella bardawil from salt soil samples by using species-specific primers and internal transcribed spacer (ITS) primers. African J Biotech. 2012;11(102):16677-87.
-
19.
Mogedas B, Casal C, Forjan E, Vilchez C. beta-carotene production enhancement by UV-A radiation in Dunaliella bardawil cultivated in laboratory reactors. J Biosci Bioeng. 2009;108(1):47-51. [PubMed ID: 19577191]. https://doi.org/10.1016/j.jbiosc.2009.02.022.
-
20.
Hosseini Tafreshi A, Shariati M. Dunaliella biotechnology: methods and applications. J Appl Microbiol. 2009;107(1):14-35. [PubMed ID: 19245408]. https://doi.org/10.1111/j.1365-2672.2009.04153.x.
-
21.
Orset S, Young AJ. Low-temperature-induced synthesis of α-carotene in the microalga Dunaliella salina (Chlorophyta). J Phycol. 1999;35(3):520-7.
-
22.
Guedes AC, Amaro HM, Pereira RD, Malcata FX. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol Prog. 2011;27(5):1218-24. [PubMed ID: 21648102]. https://doi.org/10.1002/btpr.649.
-
23.
Phadwal K, Singh PK. Effect of nutrient depletion on β-carotene and glycerol accumulation in two strains of Dunaliella sp. Bioresour Technol. 2003;90(1):55-8. https://doi.org/10.1016/S0960-8524(03)00090-7.
-
24.
Dhanam DS, Dhandayuthapani K. Optimization of-Carotene production by Marine Microalga-Dunaliella salina. Int J Curr Microbiol App Sci. 2013;2(3):37-43.
-
25.
Ben-Amotz A, Avron M. The biotechnology of cultivating the halotolerant algaDunaliella. Trends Biotechnol. 1990;8:121-6. https://doi.org/10.1016/0167-7799(90)90152-N.
-
26.
Schlipalius L. The extensive commercial cultivation of Dunaliella salina. Bioresour Technol. 1991;38(2-3):241-3. https://doi.org/10.1016/0960-8524(91)90162-D.
-
27.
Khoyi ZA, Seyfabadi J, Ramezanpour Z. Effects of light intensity and photoperiod on the growth rate, chlorophyll a and β-carotene of freshwater green micro alga Chlorella vulgaris. Comparative Biochemistry and Physiology Part A. Mol Integr Physiol. 2009;153(2):S215. https://doi.org/10.1016/j.cbpa.2009.04.601.
-
28.
Imamoglu E, Demirel Z, Dalay MC. Evaluation of culture conditions of locally isolated Dunaliella salina strain EgeMacc-024. Biochem Eng J. 2014;92:22-7. https://doi.org/10.1016/j.bej.2014.05.008.
-
29.
Borowitzka MA, Borowitzka LJ, Kessly D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J Appl Phycol. 1990;2(2):111-9. https://doi.org/10.1007/BF00023372.
-
30.
Fuggi A, Pinto G, Pollio A, Taddei R. Effects of NaCI, Na2SO4, H2SO4, and glucose on growth, photosynthesis, and respiration in the acidophilic alga Dunaliella acidophila (Volvocales, Chlorophyta). Phycologia. 1988;27(3):334-9. https://doi.org/10.2216/i0031-8884-27-3-334.1.
-
31.
Lamers PP, van de Laak CC, Kaasenbrood PS, Lorier J, Janssen M, De Vos RC, et al. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng. 2010;106(4):638-48. [PubMed ID: 20229508]. https://doi.org/10.1002/bit.22725.
-
32.
Mojaat M, Pruvost J, Foucault A, Legrand J. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem Eng J. 2008;39(1):177-8. https://doi.org/10.1016/j.bej.2007.09.009.