Abstract
Background:
Wound infection is a common problem in hospitals and is typically caused by the antibiotic-resistant Staphylococcus aureus, which is a major pathogen for skin and soft tissue infections worldwide.Objectives:
The aim of this study was to investigate the synergistic antibacterial effect of plant peptide MBP-1 and silver nanoparticles on infected wounds caused by S. aureus.Materials and Methods:
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of MBP-1 and silver nanoparticles both on their own and in combination form were determined against S. aureus via macrodilution and microdilution methods. The synergistic antibacterial effect of silver nanoparticles and MBP-1 was investigated on infected wounds caused by S. aureus in a mouse model.Results:
The MIC and MBC of MBP-1 were found to be 0.6 and 0.7 mg/mL, respectively. MIC and MBC of silver nanoparticles were determined to be 6.25 and 12.5 mg/L, respectively. MIC and MBC of the silver nanoparticles and MBP-1 combination were found to be 3.125 mg/mL, 0.5 mg/L; and 6.25 mg/mL, 0.6 mg/L, respectively. The infected wound healed properly after the combined use of MBP-1 and silver nanoparticles.Conclusions:
The synergistic effect was found on the healing of infected wounds caused by S. aureus by using an MBP-1 and silver nanoparticles combination in a mouse model.Keywords
Wound Infection Plant Peptide MBP-1 Silver Nanoparticles Antibacterial Activity Staphylococcus aureus
1. Background
Staphylococcus aureus is a very common cause of infection in hospitals and is most liable to infect newborn babies and surgical patients (1, 2). Skin infections due to S. aureus frequently begin as minor boils or abscesses, which may later progress to severe infections involving muscle or bone, and may even disseminate to the lungs or heart valves (i.e., endocarditis) (1). The capacity for S. aureus to produce human disease has not been diminished by the introduction of new antibiotics; the excessive use of antibiotics has even led to the emergence of multiple drug-resistant S. aureus strains (3).
Plants are constantly exposed to attack by a large range of pathogens. Under attack conditions, they synthesize antimicrobial peptides as their innate defense (4, 5). The antimicrobial peptides have diverse structures and functions, and they interact with the cell membranes of invader cells by disturbing the membrane integrity that leads to cell lysis and later, to their death. Pourothionins, isolated from wheat (Triticum aestivum) in 1972, was the first plant antimicrobial peptide reported to have the ability to inhibit the growth of several plant pathogens (6). Over the years, antibacterial peptides have become interesting tools in the development of new techniques for the control of crop losses, as well as in the production of novel antibiotics for the treatment of many human infections (7, 8). The antimicrobial activity of plant peptide MBP-1, isolated by Duvick et al. from Maize Kernels in 1992, has been reported as effective against both Gram-negative and Gram-positive bacteria as well as against several filamentous fungi (9).
The antimicrobial activity of silver nanoparticles (SNPs) has been reported extensively in the killing of Gram negative and Gram positive bacteria (10, 11). Silver is a more toxic element to microorganisms than many other metals, it exhibits low toxicity to mammalian cells, and has a lower propensity to induce microbial resistance than any other antimicrobial materials (12, 13).
In recent years, skin and soft-tissue infections (SSTIs) have been encountered in clinical settings with greater frequency due primarily to multidrug-resistant pathogens (14). Giacometti et al. investigated the activities of buforin II, cecropin P1, indolicidin, magainin II, and ranalexin alone and in combined form along with eight clinically used antimicrobial agents against twelve multidrug-resistant nosocomial isolates of Acinetobacter baumannii in 2000 (15). They showed that a synergistic effect occurred when magainin II was combined with β-lactam antibiotics. Ruden et al. also studied the interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides (AMPs), and reported the synergistic effect between them against Gram-negative and Gram-positive bacteria in 2009 (16).
2. Objectives
The aim of this study was to investigate the antibacterial effect of plant peptide MBP-1 and silver nanoparticles alone and in combined form against wound infections caused by S. aureus in a mouse model.
3. Materials and Methods
3.1. Material and Media
Staphylococcus aureus ATCC: 29213 was purchased from the microbial bank in Pasteur Institute, Tehran, Iran. BALB/c mice (7 - 8 weeks old and weighing 25 - 35 g) were purchased from Pasteur Institute, Tehran, Iran. Silver nanoparticles ranging in size from 3 - 18 nm and in a concentration of 4000 mg/L was purchased from NanoNasb Pars Company, Tehran, Iran. All microbial media were obtained from Merck, Germany. Plant peptide MBP-1 was purchased from BIOMATIK Company, Canada.
3.2. MIC and MBC of Silver Nanoparticles
A macrodilution method was used for determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) and was performed according to the broth macrodilution guideline from the Clinical and Laboratory Standards Institute (17). Serial dilutions of silver nanoparticles (0.78, 1.5, 3.1, 6.2, 12.5, 25, 50, 100, 200, and 400 mg/mL) were prepared in the Mueller-Hinton Broth in tubes. Then, 5 × 105 CFU/mL of S. aureus suspension was added to each tube. The samples were then incubated at 37°C and the tubes were examined for turbidity 24 hours later. The lowest concentration of silver nanoparticles that inhibited growth of S. aureus was considered as the MIC. For the calculation of the MBC, 0.1 mL of inoculum from each tube was sub-cultured on the Mueller-Hinton agar plates. The number of colonies in the Mueller-Hinton agar plates were counted and compared with the number of CFU/mL in the original inoculums 24 hours later. The lowest concentration of silver nanoparticles that could kill 99.9 percent of bacteria was determined as the MBC (13, 18).
3.3. MIC and MBC of Plant Peptide MBP-1
The microdilution method was performed to determine the MIC and MBC of plant peptide MBP-1 (15, 16). The MIC was determined in a 48-well microtiter plate according to the broth microdilution guideline by the Clinical and Laboratory Standards Institute (17). In it, 0.0125, 0.025, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 mg/mL concentrations of plant peptide MBP-1 were prepared in a double Mueller-Hinton Broth with the volume adjusted to 250 µL. Then, 5 × 105 CFU/mL of bacterial suspension was added to each well, up to 500 µL. The rest of the procedure was performed as described in section 3.2.
3.4. MIC and MBC of MBP-1 and SNPs Combination
The microdilution method was used to determine MIC and MBC for the MBP-1 and silver nanoparticles combination (15, 16). In each well of a 48-well micro titer plate, 0.0125, 0.025, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 mg/mL of plant peptide MBP-1 was combined with 0.78, 1.5, 3.1, 6.2, 12.5, 25, 50, 100, 200, and 400 mg/mL of silver nanoparticles, respectively, to prepare different concentrations in a double Mueller-Hinton Broth, with the volume adjusted to 250 µL Then, the bacterial suspension with a 0.5 McFarland turbidity standard was added to each well, up to 500 µL. The rest of procedure was performed as previously described in section 3.2.
3.5. Mouse Model
Twenty female BALB/c mice were anesthetized via injection of a mixture of xylozine (5 mg per kg body weight) and ketamin (50 mg per kg body weight) intraperitoneally. The mice were shaved on the back and the shaven area was disinfected with alcohol. Three wounds were created via a surgical blade at a length of 1 cm to the shaven area. Bacterial suspension of S. aureus, calibrated to 1.5 × 108 CFU/mL, was applied to the wounds and left undressed to the open environment. The animals were divided into 4 groups of 5 and individually kept in separate cages. The treatment was started 48 hours after the inoculation of the bacteria. Eucerin was used as the ointment base and trial ointments were prepared with the MIC concentration of silver nanoparticles, plant peptide MBP-1, and their combination. The mice were treated with MBP-1, silver nanoparticles, the MBP-1 and silver nanoparticles combination, and Eucerin alone (as the control group). All wounds were covered with special pads. The sampling of treated wounds was performed two days later using sterile swabs (19, 20). The sampling swabs were stirred in different tubes containing normal saline. Then, 10-4, 10-5, and 10-6 dilutions of samples were prepared and cultured in Mueller-Hinton agar plates. Next, after the plates were incubated at 37°C for 24 hours, the colonies were counted and the results were reported as CFU/ml (11, 20). All animal experiments were approved by the Animal Care Committee of Tarbiat Modares University, Tehran, Iran.
3.6. Statistical Analysis
The findings were analyzed by the one-way ANOVA test using SPSS (version 18) software (Illinois, USA) to compare the results of the colony counting study in vitro and in vivo. A P value of 0.05 was considered statistically significant. All tests were performed in triplicate.
4. Results
4.1. MIC and MBC
The results of the MIC and MBC studies proved the antibacterial effect of MBP-1 and silver nanoparticles alone and in combined form against S. aureus. The MIC and MBC of silver nanoparticles, MBP-1, and their combined form are shown in Table 1.
MIC and MBC of Silver Nanoparticles, Plant Peptide MBP-1, and Their Combined Form
| Treatments | MIC, mg/mL | MBC, mg/mL |
|---|---|---|
| Silver nanoparticles | 6.25 | 12.5 |
| Plant peptide MBP-1 | 0.6 | 0.7 |
| Combined form of silver nanoparticles and plant peptide MBP-1 | ||
| SNPs | 3.125 | 6.25 |
| MBP-1 | 0.5 | 0.6 |
4.2. Mouse Model
In the animal model study, S. aureus colonies were counted 24 hours after wound sampling and reported as CFU/ml. The results are shown in Table 2.
| Group | Trials | Number of Colonies |
|---|---|---|
| 1 | Plant Peptide MBP-1 | 95 × 106 ± 14 × 103 |
| 2 | Silver Nanoparticles | 82 × 105 ± 92 × 103 |
| 3 | MBP-1 and Silver Nanoparticles Combination | 79 × 104 ± 96 × 102 |
| 4 | Control (Eucerin alone) | 251 × 107 ± 34 × 105 |
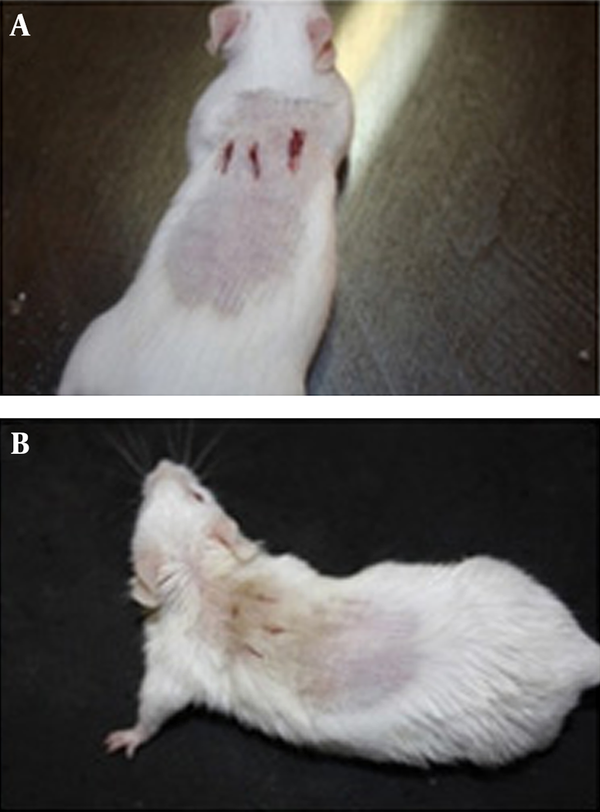
Figure 1A shows a representative mouse treated with Eucerin alone (as a control) and Figure 1B shows a representative mouse treated with the MBP-1 and silver nanoparticles combination two days after treatment. Considerable healing can be observed in the mouse treated with the combined form of MBP-1 and silver nanoparticles when compared to the control mouse.
Representative Mice Two Days After Treatment
5. Discussion
Antimicrobial peptides are widespread among a diverse range of living organisms, from bacteria to insects, plants, and animals. One of the most important advantages of antimicrobial peptides in contrast to conventional antibiotics is that they have several targets and several modes of action simultaneously (21). The antimicrobial peptides are small, cationic, and amphiphilic peptides, characterized by antimicrobial activity against bacteria, fungi, viruses, and other pathogens (4). Therefore, resistance against such antibacterial substances is apparently more difficult to form in comparison with existing antibiotics (21). However, some human pathogenic bacteria have developed resistance against human antimicrobial peptides during evolution. Knowing this, plant antimicrobial peptides may prove useful for treating infectious diseases in humans because they have had no or rare contact with human pathogens to induce any such resistance mechanisms. So far, the antimicrobial activity of plant peptide MBP-1 alone and in combination with silver nanoparticles on S. aureus has not been studied. In this study to obtain a more effective antimicrobial compound to treat infected wounds caused by S. aureus, the antibacterial effect of the combined form of plant peptide MBP-1 and silver nanoparticles was investigated.
The results of the macrodilution tests showed that silver nanoparticles have a good antimicrobial effect against S. aureus at low concentrations, which is consistent with the results of recent researches performed by Alizadeh et al. (22) and Bokaeian et al. (23). Also, the microdilution test showed the antibacterial activity of plant peptide MBP-1 against S. aureus. The results of the broth dilution method showed a strong synergistic effect between silver nanoparticles and plant peptide MBP-1. This effect had also been observed in a previous study performed in 2009 by Ruden et al. between silver nanoparticles and membrane-permeabilizing antimicrobial peptides against some Gram-negative and Gram-positive bacteria (16).
The animal study demonstrated the antibacterial activity of plant peptide MBP-1 and silver nanoparticles alone and in combined form in regards to the healing of infected wounds caused by S. aureus. There was a significant difference between the number of bacteria colonies counted in all experimental groups (treated with plant peptide MBP-1, silver nanoparticles, and their combination) and the control group (P value < 0.05). This study showed that the combination of silver nanoparticles with peptide MBP-1 increased wound healing rates and decreased the colonization of S. aureus significantly (Table 2). These results confirm the synergistic effect between MBP-1 and silver nanoparticles in healing infected wounds caused by S. aureus.
Acknowledgements
References
-
1.
McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12(11):1715-23. [PubMed ID: 17283622]. https://doi.org/10.3201/eid1211.060190.
-
2.
Ebrahimi A, Ghasemi M, Ghasemi B. Some Virulence Factors of Staphylococci Isolated From Wound and Skin Infections in Shahrekord, IR Iran. Jundishapur J Microbiol. 2014;7(4). eee9225. [PubMed ID: 25147697]. https://doi.org/10.5812/jjm.9225.
-
3.
Harris LG, Foster SJ, Richards RG. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: review. Eur Cell Mater. 2002;4:39-60. [PubMed ID: 14562246].
-
4.
Butu M, Butu A. Antimicrobial peptides-natural antibiotics. Rom Biotechnol Lett. 2011;16(3):6135-45.
-
5.
Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27-55. [PubMed ID: 12615953]. https://doi.org/10.1124/pr.55.1.2.
-
6.
Fernandez de Caleya R, Gonzalez-Pascual B, Garcia-Olmedo F, Carbonero P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Microbiol. 1972;23(5):998-1000. [PubMed ID: 5031563].
-
7.
Barbosa Pelegrini P, Del Sarto RP, Silva ON, Franco OL, Grossi-de-Sa MF. Antibacterial peptides from plants: what they are and how they probably work. Biochem Res Int. 2011;2011:250349. [PubMed ID: 21403856]. https://doi.org/10.1155/2011/250349.
-
8.
Yoganathan V. Evaluation of the effects of antimicrobial peptides on endodontic pathogens in vitro. Otago: Otago; 2012.
-
9.
Duvick JP, Rood T, Rao AG, Marshak DR. Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. J Biol Chem. 1992;267(26):18814-20. [PubMed ID: 1527010].
-
10.
Ansari MA, Khan HM, Khan AA. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MSRA on isolates from skin infections. Biol Med. 2011;3(2):141-6.
-
11.
Naqvi SZ, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomedicine. 2013;8:3187-95. [PubMed ID: 23986635]. https://doi.org/10.2147/IJN.S49284.
-
12.
Kora AJ, Arunachalam J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J Microbiol Biotech. 2011;27(5):1209-16. https://doi.org/10.1007/s11274-010-0569-2.
-
13.
Alizadeh H, Salouti M, Shapouri R. Bactericidal Effect of Silver Nanoparticles on Intramacrophage Brucella abortus 544. Jundishapur J Microbiol. 2014;7(3). eee9039. [PubMed ID: 25147682]. https://doi.org/10.5812/jjm.9039.
-
14.
Yao D, Yu FY, Qin ZQ, Chen C, He SS, Chen ZQ, et al. Molecular characterization of Staphylococcus aureus isolates causing skin and soft tissue infections (SSTIs). BMC Infect Dis. 2010;10:133. [PubMed ID: 20500885]. https://doi.org/10.1186/1471-2334-10-133.
-
15.
Giacometti A, Cirioni O, Del Prete MS, Paggi AM, D'Errico MM, Scalise G. Combination studies between polycationic peptides and clinically used antibiotics against Gram-positive and Gram-negative bacteria. Peptides. 2000;21(8):1155-60. [PubMed ID: 11035200].
-
16.
Ruden S, Hilpert K, Berditsch M, Wadhwani P, Ulrich AS. Synergistic interaction between silver nanoparticles and membrance-permeabilizing Antimicrobial peptides. Antimicrob Agents Chemother. 2009;53(8):3538-40. [PubMed ID: 19528287]. https://doi.org/10.1128/AAC.01106-08.
-
17.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Oregon; Clinical and Laboratory Standards Institute. 2014.
-
18.
Gottenbos B, van der Mei HC, Klatter F, Nieuwenhuis P, Busscher HJ. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials. 2002;23(6):1417-23. [PubMed ID: 11829437].
-
19.
Djouhri-Bouktab L, Alhanout K, Andrieu V, Raoult D, Rolain JM, Brunel JM. Squalamine ointment for Staphylococcus aureus skin decolonization in a mouse model. J Antimicrob Chemother. 2011;66(6):1306-10. [PubMed ID: 21447519]. https://doi.org/10.1093/jac/dkr114.
-
20.
Cao L, Dai C, Li Z, Fan Z, Song Y, Wu Y, et al. Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS One. 2012;7(7). eee40135. [PubMed ID: 22792229]. https://doi.org/10.1371/journal.pone.0040135.
-
21.
Aliahmadi A, Roghanian R, Emtiazi G, Mirzajani F, Ghassempour A. Identification and primary characterization of a plant antimicrobial peptide with remarkable inhibitory effects against antibiotic resistant bacteria. Sci Iran. 2012;11(40):9672-6.
-
22.
Alizadeh H, Salouti M, Shapouri R. Intra macrophage antimicrobial effect of silver nanoparticles against Brucella melitensis 16M. Sci Iran. 2013;20(3):1035-8.
-
23.
Bokaeian M, Fakheri BA, Mohasseli T, Saeidi S. Antibacterial Activity of Silver Nanoparticles Produced by Plantago Ovata Seed Extract Against Antibiotic Resistant Staphylococcus aureus. Int J Infect. 2015;2(1). eee22854.