Abstract
Background:
Diarrhea is the most frequent health problem among children in developing countries. Defining the etiology of acute diarrhea is critical to disease therapy and prevention. Some anaerobic bacteria such as Enterotoxigenic Bacteroides fragilis (ETBF) strains cause diarrheal disease by production of enterotoxin in children less than 5 years old.Objectives:
This study aimed to evaluate the prevalence of ETBF among common bacteria and viruses causing diarrhea in children aged less than five years.Materials and Methods:
One hundred diarrheal stools were cultured for detection of aerobic and anaerobic pathogen bacteria by direct plating on selective media and antibiotic susceptibility tests were performed according to clinical and laboratory standards institute (CLSI) guidelines on isolates of ETBF. The enterotoxigenic gene among B. fragilis isolates was also investigated using the polymerase chain reaction (PCR) method. Detection of viral pathogens was carried out using the latex agglutination test.Results:
Ten B. fragilis were isolated from 100 diarrheal fecal specimens. All isolates were susceptible to metronidazole, while 10% were susceptible to clindamycin. Four (40%) ETBF were isolated. Rotaviruses (57.2%) and adenoviruses (18.6%) were the most frequently detected etiological agents.Conclusions:
ETBF is one of the etiological agents that may cause diarrhea in children but it is not the commonest of them. Metronidazole is still an effective antibiotic against B. fragilis. Viruses are the most important etiological agents of diarrhea in children less than 5 years of age.Keywords
Diarrhea Susceptibility Pattern PCR Rotavirus Adenovirus Bacteroides fragilis
1. Background
Infective diarrheal disease is a major public health problem throughout the word and a leading cause of morbidity and mortality among children. It can be caused by a wide range of bacteria, viruses, and parasites (1). Every child under 5 years of age experiences about three incidents of diarrhea per year in the developing world (2). More than 200 viral, bacterial, and parasitic pathogens of diarrhea have been recognized to date, but only a few etiological agents cause the enormous majority of diarrheal diseases in children (2). These include rotavirus, adenoviruses, Escherichia coli, Campylobacter jejuni, Shigella spp., non-typhoidal Salmonella, Giardia lamblia, Cryptosporidium spp., enterotoxigenic Bacteroides fragilis and Entamoeba histolytica (2-5). Unluckily, a large number of cases of diarrheal disease are of unidentified etiology. There are several reasons for this, including weakness of causative pathogeneses, demanding growth requirements, and lack of acknowledgment of some organisms as enteric pathogens (2, 6).
Some of the anaerobic bacteria, such as B. fragilis, can also cause diarrhea in children. Bacteroides fragilis is an anaerobic Gram-negative bacillus and a member of the normal flora community of the human gastrointestinal tract and vagina. B. fragilis represents a common cause of endogenous infections in humans that is frequently associated with polymicrobial infections such as intra-abdominal, diabetic foot, obstetric-gynecologic tract and surgical site infections, as well as toxin-dependent diarrhea in adults and children (7, 8). Enterotoxigenic Bacteroides fragilis (ETBF) induced diarrhea in children has been reported by different researchers in various parts of the world, but ETBF has also been found in non-diarrheal fecal samples (9, 10).
Enterotoxigenic Bacteroides fragilis (ETBF) strains produce a 20 KDa zinc metalloprotease called fragilisyn. ETBF has been described as causing diarrheal disease in animals and particularly in human children. This toxin results in swelling and rounding of the cultured enteric cell line (11, 12).
There is evidence to confirm that antimicrobial agents can decrease the severity and duration of some intestinal infections, chiefly in those bacterial infections that cause acute watery diarrhea. There are several antibiotics that have been studied in the management of infectious diarrhea, some empirical and some targeted (13). Due to the length of time required to culture, isolate and determine the sensitivity of anaerobic isolates to different antibiotics and the high costs of these procedures, anaerobic infections are often treated empirically, based on surveillance reports of susceptibility patterns of these pathogens. Susceptibility varies considerably among the different species in the group (14).
In recent years, there has been an increasing resistance among B. fragilis to a wide range of antibacterial agents including metronidazole (MTZ), clindamycin (CD), β-lactams and others agents (15-17). The increasing resistance to different antibiotics in clinical isolates of B.fragilis leads to limitation of therapeutic alternatives in the treatment of infection caused by this organism. Susceptibility to antibiotic agents varies among anaerobic bacteria according to geographical region, and in most cases, even from one hospital to other hospitals in the same region (14).
2. Objectives
This study aimed to assess the role of ETBF among the most common pathogens causing diarrhea in children and to determine minimum inhibitory concentration (MIC) for metronidazole and clindamycin in these isolates.
3. Materials and Methods
3.1. Sampling
In a descriptive study, 100 diarrheal fecal samples were collected from outpatient and hospitalized children that were sent consecutively to the laboratory of pediatric education and medical center by physicians. Specimens were examined directly for vegetative forms of parasites and cysts by standard microscopy with iodine-stained wet-mount preparations (18). Stool samples were cultured on Hecktoen Enteric agar (Merck co, Germany) for Shigella and Salmonella strains and MacConkey agar (Merck co, Germany) for detection of E. coli. Bacterial isolates were identified according to the standard microbiological procedures (3). For isolation of B. fragilis, specimens were cultured in Bacteroides Bile Esculine agar (BBE, Himedia Laboratories Pvt. Ltd., India) and Kanamycin-Vancomycin-Laked Blood (KVLB, Basal Medium is Brucella agar (Fluka Chmie AG CH-9471 Buchs, Switzerland) media, and incubated at 37°C for 48 hours in anaerobic atmosphere (H2 = 10%, N2 = 80%, CO2 = 10%) using Mart jars (MART Microbiology, B.V. The Netherlands) and the Anoxomat system (19).
Suspicious 1 mm colonies with black surroundings (showing esculin hydrolysis) were selected in BBE medium and further identified after conducting an anaerobic tolerance test using MID8 (Mast Identification 8, according to the manufacturer’s instructions) and also some biochemical tests such as catalase and indole production and sugar fermentation (sucrose, arabinose, xylose, and rhamnose) (19).
3.2. Rotavirus and Adenovirus Latex Agglutination Test
Commercial latex agglutination test (Biomerieux, France) for the detection of Rotavirus and Adenovirus in human feces was used according to the manufacturer’s instructions. In this test latex particles, coated with monoclonal antibodies directed towards specific Rotavirus and Adenovirus antigens, cross-link in the presence of Rotavirus and Adenovirus antigens, resulting in clearly visible agglutination. In the absence of these viral antigens, the particles remain in smooth suspensions (20).
3.3. Determination of Antibiotic Sensitivity
Etest strips (Liofilchem co. Italy) were used on brucella agar supplemented with 5% sheep blood for MIC determination of metronidazole and clindamycin against B. fragilis isolates. Plates were incubated anaerobically at 37°C for 48 hours and results were interpreted by clinical and laboratory standards institute (CLSI) guidelines (21).
3.4. DNA Extraction
One loopful of cultured B. fragilis was suspended in 300 μL of TE buffer (10 mM Tris-HCL, 1 mM EDTA, pH 8.0) and placed at 80°C for 20 minutes to kill the bacteria. DNA was extracted by CTAB, SDS and proteinase K after sedimentation with isopropanol and washing with ethanol 70% extracted DNA was resolved in 100 μL TE buffer (22).
3.5. PCR Method
Specific primers of 294 bp fragment (BF1:5’dGACGGTGTATGTGATTTGTCTGAGAGA-3’ and BF2: 5’dATCCCTAAGATTTTATTATCCCAAGTA-3’ (Nedayefan co. Iran) was used for polymerase chain reaction (PCR).
DNA amplifications were performed in 20 μL volumes that contained 10 to 100 ng of DNA, 0.5 μM of each primer, in the presence of 2 mM MgCl2, 100 μ M each of dNTP, 50 μm KCl, 20 mM Tris-HCL, pH 8.4, and 2.5 U recombinant DNA polymerase (Jena Bioscinence Co., Germany). Amplification was performed in a DNA thermal cycler (Gradient Eppendorf) programmed for 94°C (seven minutes) as the initial denaturation step, followed by 35 cycles at 94°C (35seconds), 52°C (50 seconds), 72°C (55 seconds , and then 72°C for five minutes.
PCR products were electrophoresis in 1.2% agarose gel, and after staining with 0.5 μg/mL ethidium bromide they were visualized under ultraviolet (UV) light. The determination size of fragments was compared with 100 bp DNA ladder-size markers (Jena Bioscinence co. Germany) (22).
4. Results
In this study 100 diarrheal fecal samples of 55 males and 45 females obtained from outpatients and hospitalized children under the age of 5 were processed. Of these, 59% of the specimens were positive, of which 49 (83%) had one pathogen, nine (15.3%) had 2 pathogens, and one (1.7%) had 3 pathogens. Of 70 isolated pathogens, the most detected pathogen was rotaviruses 40 (57.2%), followed by adenovirus 13 (18.6%) and only 10 (14.3%) B. fragilis were isolated, four (5.72%) of which were enterotoxigenic (Table 1 and Figure 1).
Distribution of 70 Pathogens Detected from 59% Positive Specimens
| Pathogens | No. (%) |
|---|---|
| Rotavirus | 40 (57.2) |
| Adenovirus | 13 (18.6) |
| Bacteroides fragilis | 10 (14.30) |
| ETBF | 4 (5.72) |
| Non-ETBF | 6 (8.58) |
| Giardia lamblia | 3 (4.28) |
| E. coli spp. | 2 (2.85) |
| Shigella spp. | 2 (2.85) |
| Salmonella spp. | 0 |
| Total | 70 (100) |
ETBF Gene PCR Product Detected by Agarose Gel Electrophoresis (1.2%)
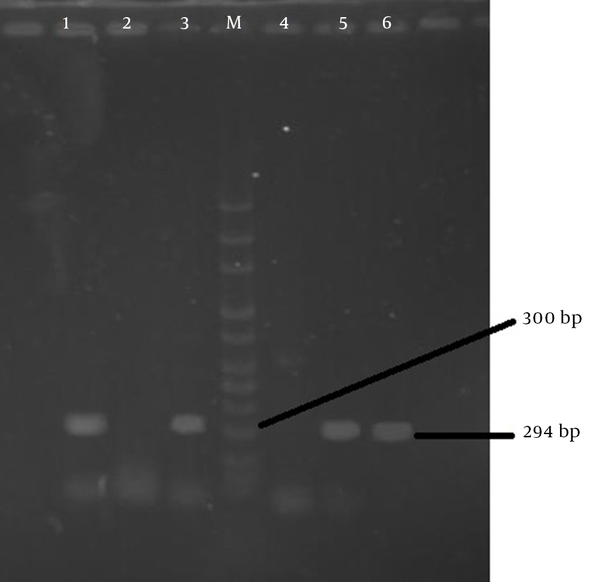
Bacteroides fragilis was isolated from children with a mean age of 2.3 years (range, 3 months to 5 years). All isolates were susceptible to MTZ, while only 10% were susceptible to clindamycin. Clinical characteristics of patients with positive culture for B. fragilis and MIC for metronidazole (0.38 - 1 μg/mL) and clindamycin (2 - 256 μg/mL) are shown in Table 2.
Clinical Characteristics of Patients and MIC Obtained for B. fragilis Isolates
| Isolate | Age | Sex | MIC (μg/mL), Obtained for | Enterotoxin Production | Hospitalization | |
|---|---|---|---|---|---|---|
| MTZ | CD | |||||
| 1 | 1 - 2 | F | 0.50, S | 2, S | Negative | In-patient |
| 2 | 2 - 3 | F | 0.50, S | 256, R | Negative | In-patient |
| 3 | 4 - 5 | M | 0.50, S | 256, R | Negative | Out-patient |
| 4 | 4 - 5 | F | 1, S | 256, R | Negative | Out-patient |
| 5 | 0 - 1 | F | 0.75, S | 256, R | Positive | In-patient |
| 6 | 1 - 2 | M | 0.75, S | 256, R | Positive | In-patient |
| 7 | 2 - 3 | M | 0.38, S | 256, R | Positive | Out-patient |
| 8 | 0 - 1 | M | 0.38, S | 256, R | Negative | Out-patient |
| 9 | 3 - 4 | M | 0.50, S | 256, R | Negative | Out-patient |
| 10 | 0 - 1 | F | 0.50, S | 256, R | Positive | Out-patient |
5. Discussion
Bacteroides fragilis usually constitutes 1% to 2% of the normal human gastrointestinal microbial flora (23). This important opportunistic obligate anaerobic pathogen commonly causes human extra-intestinal polymicrobial infections (17). Recently, the association of B. fragilis as etiological pathogen of gastrointestinal disease has been highlighted by some researchers (23). This opportunistic pathogen causes diarrheal disease via production of zinc metalloprotease enterotoxin (11).
In this study, all isolated B. fragilis studied using the PCR technique and ETBF were discovered in four (4%) cases. These findings are similar to the results of other studies carried out by Albert et al. and Jiang et al., who reported 3.5% and 4% ETBF prevalence among children with diarrheal disease (24, 25). However these results are in contrast with the results reported by Durmaz et al. in Turkey (11%) and by Niyogi et al. in India (2.3%) (26, 27).
Although some studies show that regardless of being diarrheal disease, one may be an ETBF carrier (23), Sack et al. reported 4% ETBF as an important etiologic agent in acute diarrhea in children older than 1 year in a controlled study (9).
Due to the decrease in sensitivity to antibiotics among anaerobic bacteria, selection of antibiotic drugs for treatment of infections caused by these bacteria has been difficult (23). Metronidazole has been the drug of choice for the treatment of infection caused by anaerobic bacteria worldwide for nearly 40 years but the recent emergence of metronidazole resistance is a matter of great concern (17). In our study, 100% of isolated B. fragilis were susceptible to metronidazole. These results are similar to others study carried by Nakano et al. (16). In contrast to the results obtained in our study, Akhi et al. reported 5% resistance to metronidazole among B. fragilis isolates (7). In other previous studies, metronidazole resistant Bacteroides spp. has been reported up to 15% (28). Nakano et al. also reported 34.2% resistance to metronidazole (16). Thus, quick recognition of metronidazole-resistant strains is vital for early initiation of correct antimicrobial therapy and for limiting the unsuitable administration of antibacterial drugs.
Recently there has been an increase in the resistance of bacteria to certain antibiotics such as clindamycin. In accordance with the results reported by Akhi et al. (7), we isolated 90% resistance to clindamycin. A contrasting study carried out by Seifert and Dalhoff reported 22.7% resistance to clindamycin in Germany (29). Trevino et al. also reported 45% clindamycin resistant B. fragilis in Spain (30). Thus, information regarding the prevalence of resistance among B. fragilis in each geographical region is important for the appropriate administration of antibiotics (14, 31). With the rapid increase in the frequency of clindamycin resistance in B. fragilis, this drug is no longer considered a first-line treatment for infections caused by this organism (31).
In our study, rotavirus was the commonest agent that was identified as a pathogen in cases of diarrhea, which is in agreement with other studies carried out by Vu Nguyen et al. Rotaviruses continue to be the main cause of gastroenteritis in children in industrialized areas and developing countries. The virus is an important cause of hospitalization and constant disease in children living in the U.S. and cause of death in developing countries (6).
The results obtained in this study show ETBF isolate from children with diarrheal disease in our hospitals. Metronidazole is the drug of choice for treatment of these infections, but clindamycin is an inappropriate option for empirical antibiotic therapy. Considering the increased resistance to other antibiotics, particularly metronidazole as reported by other studies, highlights the importance of determining antibiotic susceptibility patterns for appropriate antibiotic therapy. A close relationship between physicians and microbiology laboratories is essential to achieving these aims.
Acknowledgements
References
-
1.
Moyo SJ, Gro N, Matee MI, Kitundu J, Myrmel H, Mylvaganam H, et al. Age specific aetiological agents of diarrhoea in hospitalized children aged less than five years in Dar es Salaam, Tanzania. BMC Pediatr. 2011;11:19. [PubMed ID: 21345186]. https://doi.org/10.1186/1471-2431-11-19.
-
2.
Jin D, Chen C, Li L, Lu S, Li Z, Zhou Z, et al. Dynamics of fecal microbial communities in children with diarrhea of unknown etiology and genomic analysis of associated Streptococcus lutetiensis. BMC Microbiol. 2013;13:141. [PubMed ID: 23782707]. https://doi.org/10.1186/1471-2180-13-141.
-
3.
Bonkoungou IJ, Haukka K, Osterblad M, Hakanen AJ, Traore AS, Barro N, et al. Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 2013;13:36. [PubMed ID: 23506294]. https://doi.org/10.1186/1471-2431-13-36.
-
4.
Caceres M, Zhang G, Weintraub A, Nord CE. Prevalence and Antimicrobial Susceptibility of Enterotoxigenic Bacteroides fragilis in Children with Diarrhoea in Nicaragua. Anaerobe. 2000;6(3):143-8. https://doi.org/10.1006/anae.2000.0341.
-
5.
Marcos LA, DuPont HL. Advances in defining etiology and new therapeutic approaches in acute diarrhea. J Infect. 2007;55(5):385-93. [PubMed ID: 17825422]. https://doi.org/10.1016/j.jinf.2007.07.016.
-
6.
Vu Nguyen T, Le Van P, Le Huy C, Nguyen Gia K, Weintraub A. Etiology and epidemiology of diarrhea in children in Hanoi, Vietnam. Int J Infect Dis. 2006;10(4):298-308. [PubMed ID: 16458564]. https://doi.org/10.1016/j.ijid.2005.05.009.
-
7.
Akhi MT, Shirinzadeh M, Ghotaslou R, Sorous MH, Pirzadeh T, Naghavi Behzad M. Determination of Antibiotic Sensitivity of Bacteroid fragilis Isolated from Patients and Healthy Individuals in Imam Reza Center of Medical Teaching and Treatment-Tabriz. Jundishapur J Microbiol. 2013;6(9). https://doi.org/10.5812/jjm.7880.
-
8.
Rodrigues C, Siciliano RF, Zeigler R, Strabelli TM. Bacteroides fragilis endocarditis: a case report and review of literature. Braz J Infect Dis. 2012;16(1):100-4. [PubMed ID: 22358367].
-
9.
Sack RB, Albert MJ, Alam K, Neogi PK, Akbar MS. Isolation of enterotoxigenic Bacteroides fragilis from Bangladeshi children with diarrhea: a controlled study. J Clin Microbiol. 1994;32(4):960-3. [PubMed ID: 8027350].
-
10.
Merino VR, Nakano V, Liu C, Song Y, Finegold SM, Avila-Campos MJ. Quantitative detection of enterotoxigenic Bacteroides fragilis subtypes isolated from children with and without diarrhea. J Clin Microbiol. 2011;49(1):416-8. [PubMed ID: 20980581]. https://doi.org/10.1128/JCM.01556-10.
-
11.
Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva JJ. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6(2):171-4. [PubMed ID: 10756151]. https://doi.org/10.3201/eid0602.000210.
-
12.
Bressane MA, Durigon LE, Avila-Campos MJ. Prevalence of the Bacteroides fragilis Group and Enterotoxigenic Bacteroides fragilis in Immunodeficient Children. Anaerobe. 2001;7(5):277-81. https://doi.org/10.1006/anae.2001.0401.
-
13.
Casburn-Jones AC, Farthing MJ. Management of infectious diarrhoea. Gut. 2004;53(2):296-305. [PubMed ID: 14724167].
-
14.
Oteo J, Aracil B, Alos JI, Gomez-Garces JL. High prevalence of resistance to clindamycin in Bacteroides fragilis group isolates. J Antimicrob Chemother. 2000;45(5):691-3. [PubMed ID: 10797095].
-
15.
Hartmeyer GN, Soki J, Nagy E, Justesen US. Multidrug-resistant Bacteroides fragilis group on the rise in Europe? J Med Microbiol. 2012;61(Pt 12):1784-8. [PubMed ID: 22956754]. https://doi.org/10.1099/jmm.0.049825-0.
-
16.
Nakano V, Nascimento e Silva A, Merino VR, Wexler HM, Avila-Campos MJ. Antimicrobial resistance and prevalence of resistance genes in intestinal Bacteroidales strains. Clinics (Sao Paulo). 2011;66(4):543-7. [PubMed ID: 21655744].
-
17.
Chaudhary M. Emerging Metronidazole Resistance in Anaerobes and Mapping Their Susceptibility Behaviour. Am J Infect Dis. 2014;10(2):56-63. https://doi.org/10.3844/ajidsp.2014.56.63.
-
18.
Nkwengulila G, Ngoso BE, Namkinga LA. Identification of Pathogenic Intestinal Parasitic Protozoa Associated with Diarrhea among Under-fives Children in Dar Es Salaam, Tanzania. IIJMMS. 2015;2(4):49-55.
-
19.
Connie R, Mahon D, Lehman C, Manuselis G. Textbook of diagnostic microbiology. UK: WB Saunder; 2011. p. 502-37.
-
20.
Dusetty P, Velazquez FR, Gutierrez-Escolano AL, Ludert JE. Evaluation of the second generation of a commercial latex agglutination test for the detection of rotavirus antigens in fecal samples. J Clin Virol. 2013;57(1):88-90. [PubMed ID: 23403240]. https://doi.org/10.1016/j.jcv.2013.01.011.
-
21.
Methods for antimicrobial susceptibility testing of anaerobic bacteria: Approved Standard. Wayne: CLSI; 2004.
-
22.
Asgharzadeh M, Kafil HS, Khakpour M. Comparison of mycobacterial interspersed repetitive unit-variable number tandem repeat and IS6110-RFLP methods in identifying epidemiological links in patients with tuberculosis in Northwest of Iran. Ann Microbiol. 2008;58(2):333-9. https://doi.org/10.1007/bf03175339.
-
23.
Akhi MT, Ghotaslou R, Shirinzadeh M, Pirzadeh T, Naghavi Behzad M. Comparison of E test and Disk Diffusion Test for Antibiotic Resistance Testing of Enterotoxigenic and Non- Enterotoxigenic Bacteroides fragilis Isolated From Stools. Jundishapur J Microbiol. 2013;6(5). https://doi.org/10.5812/jjm.9800.
-
24.
Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37(11):3458-64. [PubMed ID: 10523534].
-
25.
Jiang ZD, Dupont HL, Brown EL, Nandy RK, Ramamurthy T, Sinha A, et al. Microbial etiology of travelers' diarrhea in Mexico, Guatemala, and India: importance of enterotoxigenic Bacteroides fragilis and Arcobacter species. J Clin Microbiol. 2010;48(4):1417-9. [PubMed ID: 20107088]. https://doi.org/10.1128/JCM.01709-09.
-
26.
Niyogi SK, Dutta P, Mitra U, Pal DK. Association of enterotoxigenic Bacteroides fragilis with childhood diarrhoea. Indian J Med Res. 1997;105:167-9. [PubMed ID: 9145599].
-
27.
Durmaz B, Dalgalar M, Durmaz R. Prevalence of enterotoxigenic Bacteroides fragilis in patients with diarrhea: a controlled study. Anaerobe. 2005;11(6):318-21. [PubMed ID: 16701593]. https://doi.org/10.1016/j.anaerobe.2005.06.001.
-
28.
Gal M, Brazier JS. Metronidazole resistance in Bacteroides spp. carrying nim genes and the selection of slow-growing metronidazole-resistant mutants. J Antimicrob Chemother. 2004;54(1):109-16. [PubMed ID: 15190033]. https://doi.org/10.1093/jac/dkh296.
-
29.
Seifert H, Dalhoff A, Prisma Study Group. German multicentre survey of the antibiotic susceptibility of Bacteroides fragilis group and Prevotella species isolated from intra-abdominal infections: results from the PRISMA study. J Antimicrob Chemother. 2010;65(11):2405-10. [PubMed ID: 20851813]. https://doi.org/10.1093/jac/dkq321.
-
30.
Trevino M, Areses P, Penalver MD, Cortizo S, Pardo F, del Molino ML, et al. Susceptibility trends of Bacteroides fragilis group and characterisation of carbapenemase-producing strains by automated REP-PCR and MALDI TOF. Anaerobe. 2012;18(1):37-43. [PubMed ID: 22261518]. https://doi.org/10.1016/j.anaerobe.2011.12.022.
-
31.
Hecht DW. Prevalence of antibiotic resistance in anaerobic bacteria: worrisome developments. Clin Infect Dis. 2004;39(1):92-7. [PubMed ID: 15206059]. https://doi.org/10.1086/421558.