Abstract
Background:
The highest incidence of listeriosis, due to consumption of ready-to-eat foods and fresh, shredded, minimally processed vegetables, occurs among pregnant women and the elderly. In order to reduce the prevalence of listeriosis among consumers, better protective measures are recommended. Chitosan films, with or without added essential oils, represent a modern, safe method of preserving the quality of such vegetables and significantly reducing the incidence of Listeria monocytogenes in these foods.Objectives:
The present study was conducted to evaluate the antimicrobial properties of composite chitosan-gelatin films with and without essential oils against two strains of L. monocytogenes, ATCC 19115 and ATCC 19112, in fresh shredded cabbage.Methods:
Shredded cabbage was inoculated with L. monocytogenes and packed between two layers of the chitosan composite film, then placed in Petri dishes. The prepared samples were stored at 4°C then analyzed for total viable count on PALCAM agar while incubated at 37°C, every 24 hours for 7 days.Results:
Average L. monocytogenes content ranged from 4.2 - 5.4 log CFU/g, reaching values of 7.2 - 8.6 log CFU/g in samples of untreated cabbage. A complete reduction of L. monocytogenes ATCC 19115 on cabbage was achieved after 120 hours in the presence of 0.5% chitosan film, whereas reduction of L. monocytogenes ATCC 19112 was achieved after 144 hours. In the presence of 1% chitosan film, the bacteria withered more quickly and complete reduction of both species of L. monocytogenes was achieved after 96 hours.Conclusions:
All tested formulations of chitosan films exhibited strong antimicrobial activity on the growth of both strains of L. monocytogenes on cabbage. The best effect was achieved with a 1% chitosan concentration. The addition of essential oils increased the antimicrobial activity of all tested films.Keywords
1. Background
Cabbage is one of the most common vegetables used in fresh salads (1). The physical and chemical characteristics of cabbage are well-known, and important for healthy nutrition. Cabbage represents an excellent source of vitamin C and a very good source of manganese. It is also a good source of polyphenols and anthocyanins, which are important as antioxidant and anti-inflammatory nutrients. This antioxidant richness and the presence of glucosinolates in cabbage are partly responsible for its cancer-prevention and cardiovascular disease-protection benefits (2).
Consumption of salad vegetables has increased significantly over the past two decades due to their health benefits. However, eating fresh vegetables carries a number of risks, especially if the vegetables are contaminated with pathogenic microorganisms (3). Among the pathogens most often associated with vegetables and salads is Listeria monocytogenes (4, 5). According to Ieren et al. (6), the highest incidence of this pathogenic microorganism occurs in cabbage, green salads, and tomatoes. The high prevalence of L. monocytogenes in fresh cabbage (95.8%) and ready-to-eat coleslaw (80.1%) in the Accra metropolis indicates that consumers of commercially prepared coleslaws have been extensively exposed to this pathogen (7). Listeria monocytogenes as a psychotropic organism is capable of growth at refrigeration temperatures, which means that low numbers of initially contaminating cells may proliferate and become hazardous if they are present on or transferred to ready-to-eat foods stored in refrigerators (8). Consequently, the consumption of minimally processed vegetables contaminated with L. monocytogenes may cause listeriosis in humans. An outbreak of listeriosis in Canada in 1981 was linked to the consumption of coleslaw contaminated with L. monocytogenes (9), and one of the largest outbreaks of listeriosis, which occurred in 2011 and included several states in the U.S., was caused by consumption of fresh cantaloupe (10).
Traditional food preservation techniques based on thermal treatments can provide microbiological stability of food, but they are not appropriate for fresh vegetables. To improve the microbiological stability of minimally processed vegetables in today’s market, researchers are looking for non-thermal treatments that do not affect the physicochemical properties or nutritional value of these products. A very popular way of preserving the quality of minimally processed, fresh-cut vegetables is the use of chitosan films (11-13). The efficacy of chitosan films has been demonstrated for different types of foods, but there is no information on the effects chitosan and the natural compounds of essential oils on cabbage.
Chitosan is a natural biodegradable polymer obtained from chitin, the second most abundant polysaccharide in nature (14). It has been used extensively in numerous industrial, health, agricultural, and food applications, and is proven to be safe (15-17). The use of chitosan as a food antimicrobial is especially attractive, primarily due to the great resistance of consumers against food that may contain chemical substances.
2. Objectives
The present study was undertaken to evaluate the survival of two strains of L. monocytogenes during storage at 4°C on packaged shredded cabbage, in the presence of chitosan-gelatin films with or without essential oils in different concentrations. The shredded vegetables were inoculated with L. monocytogenes in order to mimic contamination.
3. Methods
3.1. Sample Preparation
Fresh cabbage was purchased from local supermarkets in Sabac (Serbia) and prepared by removal of the outer leaves, then washing and shredding with a shredding machine (Multi Moulinette, Moulinex). The shredded vegetables were weighed into sterile containers in amounts of 100 g and inoculated with a prepared bacterial suspension.
3.2. Bacterial Cultures
Two pathogenic strains of L. monocytogenes, ATCC 19112 and ATCC 19115-serotype 4b, both epidemiological isolates, were used in this investigation. The selected species of bacteria originated from the ATCC (American type culture collection, Rockville, MD, USA). Before each experiment, stock cultures of bacteria were propagated through two consecutive 24-hour growth cycles in brain heart infusion agar (BHIA, HiMedia, Mumbai, India) at 37°C. Five well-isolated colonies of each strain were transferred into 10 mL of sterile saline solution (0.85% w/v) to obtain a working culture containing approximately 108 CFU mL-1. Next, 1 mL of this suspension was added to each vegetable sample (100 g each), providing an initial density in the vegetables of approximately 104 - 105 CFU g-1.
3.3. Preparation of Chitosan-Gelatin Films
Chitosan of medium molecular weight (Mw 190,000 - 310,000 Da, 75% - 85% deacetylation, Sigma-Aldrich, Germany) was used as a matrix onto which antimicrobial mint and thyme essential oils were incorporated. Chitosan was dissolved in a 1% (v/v) aqueous solution of glacial acetic acid (Merck, Germany) and stirred for 24 hours to ensure total dissolution. The final concentrations of chitosan in the film-forming solution were 0.5% (w/v), 1% (w/v), and 2% (w/v). A solution of white gelatin 6% (w/v) (Centrohem, Serbia) was prepared with 1% (v/v) of acetic acid solution using the following procedure: the gelatin was hydrated for 30 minutes at room temperature, then dissolved at 45°C (water bath) under mechanical stirring until complete dissolution (approximately 20 minutes). The prepared solutions of chitosan and gelatin were mixed in a ratio of 1:1 in order to obtain a homogenous mass that would become viscous upon cooling and create a composite film of chitosan-gelatin. As a reference, a gelatin film containing 6% (w/v) gelatin was prepared.
Approximately 25 mL of chitosan-gelatin solution was prepared for each Petri dish, of which one half was poured into the cover while the other half was poured onto the bottom of the Petri dish. After spilling of the solution, a composite film was allowed to dry at a room temperature under sterile conditions. Until use, films were stored at 4°C. The same procedure was applied to prepare chitosan-gelatin solutions containing mint or thyme essential oils (Herba Doo, Serbia), which were added to the chitosan-gelatin solution and mixed for 5 minutes in order to obtain a homogenous mass. The final concentration of tested essential oil in the coating formulations was 0.2% (w/v). To ensure emulsification of the essential oils, dimethyl sulfoxide 0.2% (w/v) (Carlo Erba Reagents, France), was added to each composite film (18).
3.4. Application of Chitosan Films
The films were applied to fresh shredded cabbage samples (40 g) using the following procedure: vegetable samples were inoculated with the bacterial suspension and packed between two layers of the composite chitosan film in Petri dishes. The prepared samples were sealed in plastic bags and stored at 4°C until analysis, which was carried out every 24 hours for 6 days.
3.5. Microbial Analysis of Vegetables Samples During Refrigerated Storage
The total number of tested bacteria was evaluated periodically throughout the storage period. Treated and untreated shredded vegetables (10 g samples) were subjected to microbial analysis during refrigerated storage at 4°C at the initial point (0 days) and at 1, 2, 3, 4, 5, and 6 days. Under sterile conditions, 10 g of each sample was homogenized for 10 minutes at 150 rpm, using a laboratory shaker (J. P. Selecta, model 3000974), in 90 mL of sterile saline solution (0.85 % NaCl w/v). Serial dilutions of each suspension were prepared and plated on Petri dishes containing PALCAM agar (Hi Media, Mumbai, India), which contained acriflavine hydrochloride (5 mg l-1), polymyxin B (10 mg 1-1), and ceftazidime (20 mg l-1) (19). The Petri plates were incubated for 24 hours at 37°C.
3.6. Statistical Analysis
All experiments were replicated three times. Results are expressed as mean values of three repetitions. Microbiological counts were converted to log CFU g-1 and statistical differences were determined using Student’s t-test (Microsoft Excel, 2007) after the analysis of variance. Significance was set at P < 0.05. In order to determine the correlation between two tested bacterial strains, Pearson’s correlation coefficient was calculated.
4. Results
The antimicrobial effect of 0.5% chitosan films against L. monocytogenes ATCC 19115 on shredded cabbage samples was evaluated during 6 days of refrigerated storage at 4°C (Figure 1). The number of L. monocytogenes ATCC 19115 increased from 4.6 log10 CFU g-1 to the 7.5 log10 CFU g-1 in the control sample of untreated cabbage. Contact of L. monocytogenes ATCC 19115 with 0.5% chitosan films in shredded cabbage resulted in rapid inhibition of growth compared to the control. Complete inhibition of growth in the presence of 0.5% chitosan films was achieved at 120 hours of storage (Figure 1).
Growth of L. monocytogenes ATCC 19115 on Cabbage at 4°C in the Presence of 0.5% Chitosan Films with and without Essential Oils
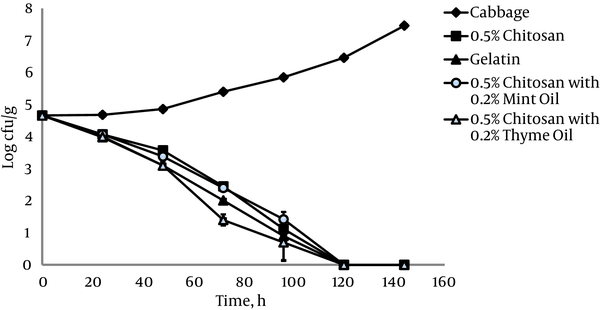
The antimicrobial effect of 0.5% chitosan films against L. monocytogenes ATCC 19112 on shredded cabbage is shown in Figure 2. Significantly increased populations on cabbage did occur with storage at 4°C, whereas significant decreases in L. monocytogenes ATCC 19112 populations occurred in shredded cabbage stored in the presence of 0.5% chitosan films.
Growth of L. monocytogenes ATCC 19112 on Cabbage at 4°C in the Presence of 0.5% Chitosan Films with and without Essential Oils
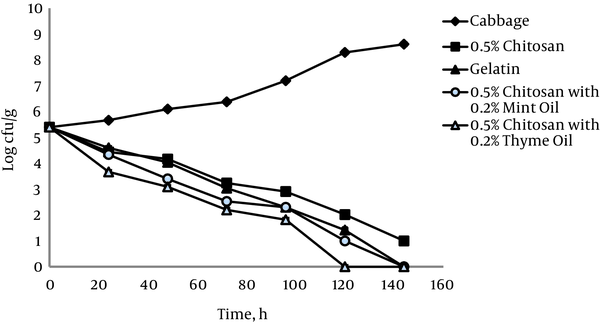
The relationship between tested strains of bacteria showed positive and statistically significant coefficient correlations, r = 0.97 and r = 0.96 (P < 0.05) in cabbage alone and in cabbage with 0.5% chitosan film, respectively.
A large amount of L. monocytogenes ATCC 19115 was present in the control shredded cabbage at the beginning of the experiment (Figure 3), and the number significantly increased during storage (P < 0.05). Using a 4.7 log initial inoculation level, treatment with 1% chitosan film for the first 24 hours resulted in a decrease of 1.3 log CFU/g compared to untreated cabbages (Figure 3). However, the same treatment achieved complete inactivation of all L. monocytogenes ATCC 19115 cells on cabbage after 120 hours.
Growth of L. monocytogenes ATCC 19115 on Cabbage at 4°C in the Presence of 1% Chitosan Films with and without Essential Oils
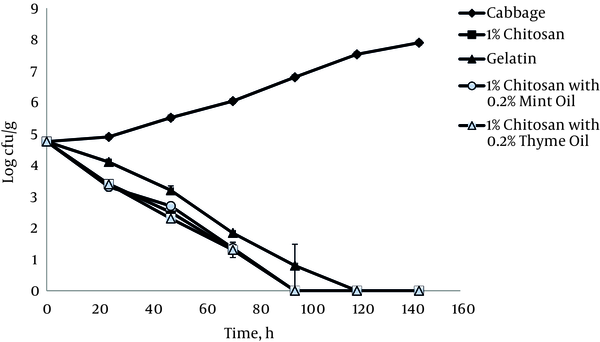
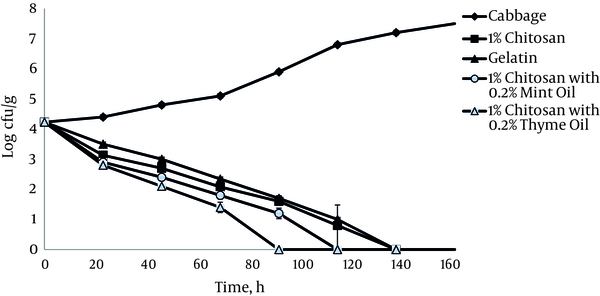
During 6 days of storage at 4°C, in the presence of 1% chitosan film, L. monocytogenes ATCC 19112 populations inoculated onto shredded cabbage decreased from 4.2 log10 CFU g-1 to undetectable levels after 144 hours (Figure 4). It was observed that the addition of thyme and mint essential oils at a concentration of 0.2% increased the antimicrobial activity of 1% chitosan films in both tested bacterial strains. The relationship between the strains showed positive and statistically significant correlation coefficients, r = 0.98 and r = 0.94 (P < 0.05) in cabbage alone and in cabbage with 1% chitosan film, respectively.
Growth of L. monocytogenes ATCC 19112 on Cabbage at 4°C in the Presence of 1% Chitosan Films with and without Essential Oils
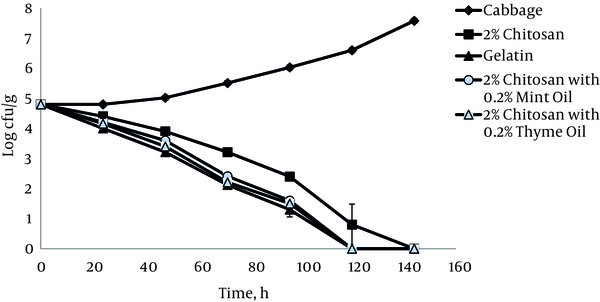
Cabbage samples treated with 2% chitosan films showed a significant reduction of the L. monocytogenes ATCC 19115 population (P < 0.05). In the presence of 2% chitosan films, microbial reduction of 1.1 log10 CFU g-1 appeared after 48 hours of storage, and there were no detectable bacteria after 6 days of refrigerated storage (Figure 5). It should be noted that in this case, application of gelatin with 1% acetic acid can yield a higher reduction in the population of L. monocytogenes ATCC 19115. The combined treatment of 2% chitosan films and essential oils showed a significant reduction (P < 0.05) of the tested bacteria, but this activity was still lower than that achieved with acetic acid alone.
Growth of L. monocytogenes ATCC 19115 on Cabbage at 4°C in the Presence of 2% Chitosan Films with and without Essential Oils
5. Discussion
Our investigation showed that L. monocytogenes can remain viable on raw shredded cabbage for periods extending beyond their normal expected shelf-life. Manufacturers usually declare the shelf-life of minimally processed cabbage to be 4 - 5 days, during which time L. monocytogenes in shredded cabbage can multiply to levels dangerous for humans. The results obtained in the present study are consistent with the findings of Monge and Arias-Echandi (20), who reported cabbage as the most common vegetable showing this organism. This could be attributed to high levels of fermentable sugars, such as glucose, which can be readily utilized by L. monocytogenes (21).
Contact of L. monocytogenes cells with all tested chitosan films resulted in decreased bacterial populations. The mechanism of this antimicrobial activity can be explained in various ways (22, 23). Some authors have suggested that the interaction between positively-charged chitosan molecules and negatively-charged microbial surfaces results in the disruption of cell membranes, leakage of intracellular constituents, and ultimately, microbial cell death (24). Additionally, according to a second hypothesis, chitosan oligomers can penetrate into prokaryotic cells and interfere with the transcription of RNA and protein synthesis (25).
The inhibition effect of chitosan films can be attributed in part to a lower pH and to acetic acid, which is used in preparing films. Other authors have reported that the type of acid, molarity, and temperature, as well as pH level, influence the ability of L. monocytogenes to grow (26). At the same pH, acetic acid was more effective than lactic, citric, malic, and hydrochloric acids. Acetic acid is the predominant acid in films and undoubtedly had a lethal effect on the two test strains of L. monocytogenes examined in this study.
Our results show that chitosan films possess antimicrobial activity that depends on the concentration of the applied films. In this study, 1% chitosan films exhibited greater antimicrobial activity compared to 0.5% chitosan films. The present results indicate that films with higher concentrations of chitosan have more pronounced antimicrobial activity, consistent with previously published data (27). However, it can be seen that 2% chitosan films exhibit less inhibition of bacterial species than 1% chitosan films. This behavior can be explained by the extremely high viscosity of the chitosan solution. According to Hirano and Nago (28), due to high viscosity, chitosan slightly diffuses through the gel, and activity is consequently decreased. The antimicrobial effect of chitosan films was even more pronounced with the addition of essential oils in this study, which is in agreement with the findings of other authors (29-31). Essential oils, due to their antimicrobial activities and safety, have been designated as GRAS (generally recognized as safe) by the U.S. Food and drug administration (32). Comparison of the effect of mint and thyme essential oils against L. monocytogenes in cabbage in the present study showed that the addition of 0.2% thyme essential oil significantly (P < 0.05) affected the antimicrobial activity of films, compared with chitosan films alone.
On the basis of our results, chitosan-gelatin films have the potential to be used in the food industry as active packaging materials to inhibit foodborne pathogens. Chitosan and essential oils may have a significant impact on the antimicrobial effect of these films. The most prominent activity was shown with 1% chitosan films and 0.2% thyme essential oil.
References
-
1.
Mritunjay SK, Kumar V. Potential Hazards of Microbial Contamination Associated with Raw Eaten Salad Vegetables and Fresh Produces. Middle East J Sci Res. 2015;23(4):741-9. https://doi.org/10.5829/idosi.mejsr.2015.23.04.22010.
-
2.
Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2008;8(1):269-82. https://doi.org/10.1007/s11101-008-9103-7.
-
3.
Ryu JH, Kim M, Kim EG, Beuchat LR, Kim H. Comparison of the microbiological quality of environmentally friendly and conventionally grown vegetables sold at retail markets in Korea. J Food Sci. 2014;79(9):1739-44. [PubMed ID: 25124136]. https://doi.org/10.1111/1750-3841.12531.
-
4.
Carrasco E, Perez-Rodríguez F, Valero A, Garcia-Gimeno RM, Zurera G. Risk Assessment and Management ofListeria Monocytogenesin Ready-to-Eat Lettuce Salads. Comprehensive Rev Food Sci Food Safety. 2010;9(5):498-512. https://doi.org/10.1111/j.1541-4337.2010.00123.x.
-
5.
Severino R, Vu KD, Donsi F, Salmieri S, Ferrari G, Lacroix M. Antimicrobial effects of different combined non-thermal treatments against Listeria monocytogenes in broccoli florets. J Food Eng. 2014;124:1-10. https://doi.org/10.1016/j.jfoodeng.2013.09.026.
-
6.
Ieren II, Bello M, Kwaga JKP. Occurrence and antibiotic resistance profile of Listeria monocytogenes in salad vegetables and vegetable salads sold in Zaria, Nigeria. Afr J Food Sci. 2013;7(9):334-8. https://doi.org/10.5897/ajfs2013.1036.
-
7.
Dogbe EE. Risk of Listeria monocytogenes ingestion in consuming coleslaw purchased from food vendors in the Accra metropolis [Dissertation]. Ghana: Department of Nutrition and Food Science, University of Ghana; 2010.
-
8.
Maktabi S, Jamnejad A, Faramarzian K. Contamination of Household Refrigerators by Listeria Species in Ahvaz, Iran. Jundishapur J Microbiol. 2013;6(3):301-5. https://doi.org/10.5812/jjm.3543.
-
9.
Schlech WF, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, et al. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983;308(4):203-6. [PubMed ID: 6401354]. https://doi.org/10.1056/NEJM198301273080407.
-
10.
Centers for Disease Control and prevention. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe--United States, August-September 2011. MMWR Morb Mortal Wkly Rep. 2011;60(39):1357-8. [PubMed ID: 21976119].
-
11.
Prajapati B. Chitosan A Marine Medical Polymer And Its Lipid Lowering Capacity. Int J Health. 2009;9(2). https://doi.org/10.5580/1045.
-
12.
Sanchez-Gonzalez L, Pastor C, Vargas M, Chiralt A, Gonzalez-Martínez C, Chafer M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol Technol. 2011;60(1):57-63. https://doi.org/10.1016/j.postharvbio.2010.11.004.
-
13.
Perdones A, Sanchez-Gonzalez L, Chiralt A, Vargas M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Food Chem. 2012;70:32-41. [PubMed ID: 26617043]. https://doi.org/10.1016/j.foodchem.2015.11.054.
-
14.
Zivanovic S, Li J, Davidson PM, Kit K. Physical, mechanical, and antibacterial properties of chitosan/PEO blend films. Biomacromolecules. 2007;8(5):1505-10. [PubMed ID: 17388625]. https://doi.org/10.1021/bm061140p.
-
15.
Gao P, Zhu Z, Zhang P. Effects of chitosan-glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr Polym. 2013;95(1):371-8. [PubMed ID: 23618282]. https://doi.org/10.1016/j.carbpol.2013.03.029.
-
16.
Wu T, Zivanovic S, Draughon FA, Conway WS, Sams CE. Physicochemical properties and bioactivity of fungal chitin and chitosan. J Agric Food Chem. 2005;53(10):3888-94. [PubMed ID: 15884813]. https://doi.org/10.1021/jf048202s.
-
17.
Bourtoom T. Edible films and coatings: characteristics and properties. Int Food Res J. 2008;15(3):237-48.
-
18.
Zivanovic S, Chi S, Draughon AF. Antimicrobial Activity of Chitosan Films Enriched with Essential Oils. J Food Sci. 2005;70(1):45-51. https://doi.org/10.1111/j.1365-2621.2005.tb09045.x.
-
19.
Severino R, Vu KD, Donsi F, Salmieri S, Ferrari G, Lacroix M. Antibacterial and physical effects of modified chitosan based-coating containing nanoemulsion of mandarin essential oil and three non-thermal treatments against Listeria innocua in green beans. Int J Food Microbiol. 2014;191:82-8. [PubMed ID: 25255308]. https://doi.org/10.1016/j.ijfoodmicro.2014.09.007.
-
20.
Monge R, Arias-Echandi ML. Presence of Listeria monocytogenes in fresh salad vegetables. Rev Biomed. 1999;10(1):29-31.
-
21.
Beuchat LR, Brackett RE, Hao DY, Conner DE. Growth and thermal inactivation of Listeria monocytogenes in cabbage and cabbage juice. Can J Microbiol. 1986;32(10):791-5. [PubMed ID: 3098397].
-
22.
Tharanathan RN, Kittur FS. Chitin--the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr. 2003;43(1):61-87. [PubMed ID: 12587986]. https://doi.org/10.1080/10408690390826455.
-
23.
Vishu Kumar AB, Varadaraj MC, Lalitha RG, Tharanathan RN. Low molecular weight chitosans: preparation with the aid of papain and characterization. Biochim Biophys Acta. 2004;1670(2):137-46. [PubMed ID: 14738997].
-
24.
Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51-63. [PubMed ID: 20951455]. https://doi.org/10.1016/j.ijfoodmicro.2010.09.012.
-
25.
Hafdani FN, Sadeghinia N. A review on application of chitosan as a natural antimicrobial. World Acad Sci Eng Technol. 2011;5(2):46-50.
-
26.
Sorrells KM, Enigl DC, Hatfield JR. Effect of pH, acidulant, time, and temperature on the growth and survival of Listeria monocytogenes. J Food Protect. 1989;8:571-3.
-
27.
Zhang H, Li R, Liu W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: a review. Int J Mol Sci. 2011;12(2):917-34. [PubMed ID: 21541034]. https://doi.org/10.3390/ijms12020917.
-
28.
Hirano S, Nagao N. Effects of Chitosan, Pectic Acid, Lysozyme, and Chitinase on the Growth of Several Phytopathogens. Agric Biol Chem. 2014;53(11):3065-6. https://doi.org/10.1080/00021369.1989.10869777.
-
29.
Petrou S, Tsiraki M, Giatrakou V, Savvaidis IN. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int J Food Microbiol. 2012;156(3):264-71. [PubMed ID: 22534355]. https://doi.org/10.1016/j.ijfoodmicro.2012.04.002.
-
30.
Vu KD, Hollingsworth RG, Leroux E, Salmieri S, Lacroix M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res Int. 2011;44(1):198-203. https://doi.org/10.1016/j.foodres.2010.10.037.
-
31.
Sánchez-Gonzalez L, Cháfer M, Chiralt A, González-Martínez C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohyd Pol. 2010;82(2):277-83. https://doi.org/10.1016/j.carbpol.2010.04.047.
-
32.
Vyry Wouatsa NA, Misra L, Venkatesh Kumar R. Antibacterial activity of essential oils of edible spices, Ocimum canum and Xylopia aethiopica. J Food Sci. 2014;79(5):972-7. [PubMed ID: 24758511]. https://doi.org/10.1111/1750-3841.12457.