Abstract
Background:
Toxoplasmosis is a life-threatening infection in organ transplant recipients, people receiving corticosteroid or radiation therapy, people with malignancies, and AIDS patients.Objectives:
The current study aimed to determine the prevalence of toxoplasmosis in patients receiving chemotherapy for malignancies in the Bushehr province of southwest Iran.Methods:
Blood samples were taken from 86 patients who were continuously referred to the chemotherapy center in Bushehr province and evaluated by ELISA to determine anti-Toxoplasma IgG and IgM antibodies. Moreover, a blood buffy coat of each sample was assessed by polymerase chain reaction (PCR), targeting a 529 bp gene of T. gondii. PCR products of the positive samples were sequenced to determine the genotype of the parasite.Results:
Anti-Toxoplasma IgG antibodies were detected in the sera of 21 (24.4%) cases. All of the patients were negative for anti-Toxoplasma IgM antibodies. No statistically significant correlation was found between seropositivity to Toxoplasma and duration of chemotherapy or having contact with cats. PCR detected a 529 bp band of T. gondii in the buffy coats of two out of 86 (2.3%) cases. The sequence analysis demonstrated that both cases were 95% identical to type III (VEG strain) of T. gondii.Conclusions:
Findings of this study demonstrated the presence of type III T. gondii in the buffy coats of patients undergoing chemotherapy. Given that toxoplasmosis is a life-threatening infection in immunocompromised patients, these patients should be screened for toxoplasmosis before and during chemotherapy to prevent acute toxoplasmosis.Keywords
Toxoplasmosis Seroprevalence Chemotherapy Bushehr Province Iran
1. Background
Toxoplasmosis is a zoonotic infection caused by an intracellular protozoan, Toxoplasma gondii, which can infect humans and a wide range of animals (1). The seroprevalence of toxoplasmosis in the human population varies depending on geographical and socioeconomic conditions, including eating habits, health-related practices, and host susceptibility (2-4). The lowest rate of infection can be seen in warm, dry, and cold areas, whereas the prevalence of infection in tropical areas with humid and warm climates is high (1).
Toxoplasmosis usually causes no overt signs or symptoms in immunocompetent individuals. In such cases, mild flu-like illness with relatively common symptoms such as muscle aches and tender lymph nodes may be infrequently observed. However, toxoplasmosis is a serious opportunistic infection of immunocompromised patients, including those with different type of malignancies, people receiving long-term steroid therapy or cytotoxic drugs, and AIDS patients (5, 6). Toxoplasma latency occurs after acquired infection in which the parasite remains in the body as a latent infection in the form of cysts in the skeletal muscle, cardiac muscle, and/or the brain, which are usually inactive and harmless. Reactivation occurs only in immunodeficient patients.
Toxoplasma gondii is classified into three different genotypes (I, II, and III) based on isoenzyme electrophoresis patterns and molecular differences (7). These three clonal lineages may have differences in virulence, pathogenicity, and epidemiological patterns of occurrence. Thus, detection of Toxoplasma infection in patients with any immunodeficiency status and determination of the genotype of the parasite is important in the successful management of the disease.
2. Objectives
Considering the importance of toxoplasmosis in immunocompromised patients, this study was carried out to determine the seroprevalence of toxoplasmosis specifically the genotype of T. gondii in people undergoing chemotherapy in the Bushehr Province of southwest Iran, which is located at the edge of the Persian Gulf and experiences hot, humid weather during most seasons of the year.
3. Methods
3.1. Study Population and Sample Collection
Subjects of this cross-sectional study were 86 patients who were referred from December, 2014 to July, 2015 to the chemotherapy center in Bushehr province. Ethical approval for the study was given by the ethics committee of the Shiraz University of Medical Sciences (ethics committee code: IR.SUMS.REC.1394.S350). Informed consent (oral) was obtained from the participants (or their parents, in the case of minors), and confidentiality of the participants’ information was guaranteed.
Blood samples (5 mL) were taken from each subject, and sera were obtained from the collected blood. In addition, a buffy coat was obtained from each blood sample for subsequent molecular evaluation. Sera and buffy coats were kept at -20°C until use. Sociodemographic information and data related to the prevalence of toxoplasmosis including patient’s residence, duration of chemotherapy, educational level, contact with animals, and keeping of house cats were collected through a predesigned questionnaire.
3.2. Serological Evaluation
Sera samples were tested for IgG and IgM anti-Toxoplasma antibodies using a commercial ELISA kit (PishtazTeb Diagnostics, Tehran, Iran) based on the manufacturer’s instructions.
3.3. DNA Extraction and PCR Amplification
DNA was extracted from the buffy coat of each sample, using the phenol chloroform method as previously described (8). PCR was performed to amplify a 529 bp gene of T. gondii, as described by Edvinsson et al. (9), using each of two primers: TOXOF CAGGGAGGAAGACGAAAGTTG and TOXOR CAGACACAGTGCATCTGGATT. The PCR reaction mixture (25 µL) contained 1.25 units of Taq DNA polymerase, 1 μL of extracted DNA, 1.5 mM of MgCl2, 100 pmol of each primer, 0.2 mM of dNTP and a 10x PCR buffer. The PCR program consisted of one cycle of initial denaturation at 94°C for 5 minutes, 35 cycles of denaturation at 94°C for 35 seconds, annealing at 56°C for 1 minute, extension at 72°C for 1 minute, and a final extension at 72°C for 10 minutes. PCR products were separated by electrophoresis in 1.5% agarose gel and stained with ethidium bromide. The PCR product was excised from the agarose gel and purified using a DNA Gel Extraction Kit (Bioneer’s AccuPrep Gel Purification Kit, Korea), based on the manufacturer’s instructions. The purified PCR product was sequenced using the same primers used for PCR amplification. BLAST analysis was used to compare the sequences with those of available T. gondii sequences in the GenBank to discover the genotype of the parasite.
3.4. Statistical Analysis
Collected data were analyzed using statistical package for the social sciences (SPSS) software (SPSS Inc., Chicago, IL, USA; version 18,). The prevalence values relative to the features of the subjects were analyzed with a chi-square or Fisher's exact test.
4. Results
Of the 86 patients studied, 37 (43%) were male and 49 (57%) were female. Most of the subjects were aged 40 - 60 years. Anti-Toxoplasma IgG was detected in 21 (24.4%) out of 86 patients. None of the subjects tested had anti-Toxoplasma IgM antibodies. Statistical analysis showed no significant correlation between seropositivity to Toxoplasma and patient age (P > 0.05).
The frequency of anti-T. gondii IgG antibodies was higher in women (12.8%) than in men (11.6%), but this difference was not statistically significant (P > 0.05). Of the 59 patients residing in urban areas, 13 of them (15.1%) were seropositive for anti-Toxoplasma IgG antibodies, and this prevalence rate was 9.3% for those living in rural areas. The prevalence of anti-Toxoplasma IgG antibodies in illiterate patients was higher than in educated people, although this difference was not statistically significant. Table 1 shows the sociodemographic features of the patients and their relative seropositivity to Toxoplasma in this study.
Sociodemographic Features of the Patients Receiving Chemotherapy and Relative Seropositivity to T. gondii in Bushehr Province
| Characteristics | Frequency (No.) | Percent (%) | Positive for anti-Toxoplasma Antibody (IgG), No. % | P Value |
|---|---|---|---|---|
| Age group, Y | > 0.05 | |||
| ≤ 20 | 2 | 2.3 | 0 | |
| 21 - 40 | 21 | 24.4 | 6 (7) | |
| 41 - 60 | 41 | 47.7 | 11 (12.8) | |
| > 61 | 22 | 25.6 | 4 (4.7) | |
| Gender | > 0.05 | |||
| Male | 37 | 43 | 10 (11.6) | |
| Female | 49 | 57 | 11 (12.8) | |
| Educational level | > 0.05 | |||
| Illiterate | 41 | 47.7 | 13 (15.1) | |
| Low literacy | 13 | 15.1 | 2 (2.3) | |
| Educated | 32 | 37.2 | 6 (7) | |
| Residence | > 0.05 | |||
| Urban | 59 | 68.6 | 13 (15.1) | |
| Rural | 27 | 31.4 | 8 (9.3) | |
| Contact with animals | > 0.05 | |||
| Yes | 47 | 54.7 | 10 (12.8) | |
| No | 39 | 45.3 | 11 (11.6) | |
| Keeping cat | > 0.05 | |||
| Yes | 22 | 25.6 | 4 (4.7) | |
| No | 64 | 74.4 | 17 (19.8) | |
| Treatment duration, Months | > 0.05 | |||
| < 3 | 16 | 18.7 | 4 ( 4.7) | |
| 3 - 6 | 28 | 32.5 | 7 (8.1) | |
| 6 - 12 | 32 | 37.2 | 7 (8.1) | |
| > 12 | 10 | 11.6 | 3 (3.5) |
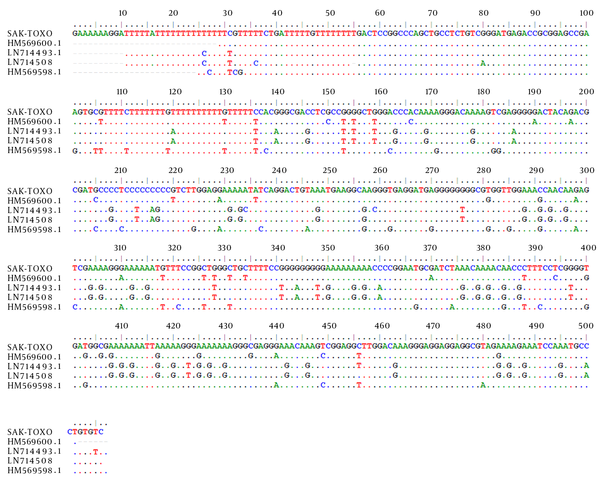
All the patients’ buffy coat samples were tested for the presence of T. gondii DNA by PCR. PCR detected a 529 bp band of T. gondii in the buffy coats of two out of 86 (2.3%) cases. Of these two cases, one was seropositive for IgG and the other was seronegative. Sequence analysis demonstrated that the cases were 95% identical to those of available sequences in GenBank for type III (VEG) of T. gondii (Figure 1).
Alignment of a Sequence of Toxoplasma Isolated From the Buffy Coat of a Patient Undergoing Chemotherapy for Malignancies
5. Discussion
Toxoplasmosis is usually an asymptomatic infection in adult humans, but it can be a fatal disease in immunocompromised patients. This disease is life threatening in organ transplant recipients, people receiving corticosteroid or radiation therapy, people with malignancies, and AIDS patients (5). Clinical manifestations in these patients occur due to reactivation of latent toxoplasmosis and can lead to fatal meningoencephalitis and focal lesions in the central nervous system, although they are less likely to cause myocarditis and pneumonia (1, 5, 10).
In the present study, the prevalence of anti-T. gondii antibodies in patients undergoing chemotherapy in Bushehr province was found to be 24.4%. This means that about one quarter of the patients receiving immunosuppressant drugs have latent toxoplasmosis, and there is always a risk of reactivation of Toxoplasma in these patients. Other studies that have been conducted in different parts of Iran with different geographical conditions and on patients with different sociodemographic characteristics have also documented a relatively high prevalence of toxoplasmosis in such patients (5, 11). In a systematic review and meta-analysis study on 2,800 Iranian immunocompromised patients, the overall seroprevalences of toxoplasmosis in AIDS patients, patients receiving transplant organs, and cancer patients have been reported as 50.5, 55.1, and 45.6%, respectively (5). A study on patients with malignancies in Ahwaz, south Iran revealed a seroprevalence rate of 45.2% for toxoplasmosis (11).
The seroprevalence rate of toxoplasmosis in patients undergoing chemotherapy in the current study is lower than that of patients living in other areas of the country (5, 11). In fact, previous studies conducted on healthy people in Bushehr Province have revealed a lower seroprevalence of toxoplasmosis in comparison with other parts of the country. In a study in 2010 by Fouladvand et al. (12), the prevalence of toxoplasmosis in high school girls in Bushehr was reported to be 11.5%. Bushehr is a tropical region located in southwest Iran, and the temperature in this area is much higher than in other parts of the country during most seasons of the year. The climate and weather of the region do not appear favorable to the growth and transmission of Toxoplasma oocysts (12-14). In addition, individuals indigenous to this region tend to eat more seafood than beef or lamb and cook the meat properly. As a result, these individuals are less prone to infection through consumption of tissue cysts of T. gondii, which is considered one of the main sources of Toxoplasma infection in Iran (13, 14).
In the current study, the relationship between residence location and toxoplasmosis prevalence in studied patients was not statistically significant. In recent years, increasing improvements have been made in the health facilities and cultural development in rural areas of Iran that have transformed the relatively traditional rural lifestyle into a relatively modern urban life. These lifestyle changes in turn have reduced the chance of transmission of zoonotic infections, although infection in animals is still common (13-15).
In the present study, PCR was used to detect T. gondii infection in the buffy coats of patients. PCR detected Toxoplasma DNA in the buffy coat of two cases, one seropositive and the other seronegative. Sequences of the isolates were most similar to type III of the T. gondii genotypes. The presence of type I T. gondii was previously reported in cases of congenital toxoplasmosis in southwest Iran (16). In Spain, T. gondii type II was found to be the most prevalent (52%) genotype in immunocompromised patients (17).
A Study of Khan et al. (2005) on the cerebral spinal fluid of HIV-positive patients revealed that a majority of these patients were infected with type I strains of T. gondii (18). On the other hand, genotyping analysis of T. gondii isolates in samples collected from 88 immunocompromised patients from European countries revealed Type III T. gondii as the second most common genotype recovered from patients. The authors of the study concluded that host factors are much more involved than parasite factors in patients’ resistance or susceptibility to toxoplasmosis (19).
Taken together, the findings of the current study demonstrated T. gondii infection in about one quarter of the patients undergoing chemotherapy in the Bushehr province of southwestern Iran. Because toxoplasmosis is an important life-threatening infection in immunocompromised patients, these patients should be screened for toxoplasmosis before chemotherapy, as well as during treatment for the prevention of acute toxoplasmosis.
Acknowledgements
References
-
1.
Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264-96. [PubMed ID: 22491772]. https://doi.org/10.1128/CMR.05013-11.
-
2.
Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. 2014;137:185-94. [PubMed ID: 24887263]. https://doi.org/10.1016/j.actatropica.2014.05.015.
-
3.
Flegr J, Prandota J, Sovickova M, Israili ZH. Toxoplasmosis--a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9(3):90203. [PubMed ID: 24662942]. https://doi.org/10.1371/journal.pone.0090203.
-
4.
Sarkari B, Shafiei R, Zare M, Sohrabpour S, Kasraian L. Seroprevalence and molecular diagnosis of Toxoplasma gondii infection among blood donors in southern Iran. J Infect Dev Ctries. 2014;8(4):543-7. [PubMed ID: 24727522]. https://doi.org/10.3855/jidc.3831.
-
5.
Ahmadpour E, Daryani A, Sharif M, Sarvi S, Aarabi M, Mizani A, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries. 2014;8(12):1503-10. [PubMed ID: 25500647]. https://doi.org/10.3855/jidc.4796.
-
6.
Scerra S, Coignard-Biehler H, Lanternier F, Suarez F, Charlier-Woerther C, Bougnoux ME, et al. Disseminated toxoplasmosis in non-allografted patients with hematologic malignancies: report of two cases and literature review. Eur J Clin Microbiol Infect Dis. 2013;32(10):1259-68. [PubMed ID: 23595587]. https://doi.org/10.1007/s10096-013-1879-8.
-
7.
Cristina N, Darde ML, Boudin C, Tavernier G, Pestre-Alexandre M, Ambroise-Thomas P. A DNA fingerprinting method for individual characterization of Toxoplasma gondii strains: combination with isoenzymatic characters for determination of linkage groups. Parasitol Res. 1995;81(1):32-7. [PubMed ID: 7724511].
-
8.
Sarkari B, Gadami F, Shafiei R, Motazedian MH, Sedaghat F, Kasraian L, et al. Seroprevalence of Leishmania infection among the healthy blood donors in kala-azar endemic areas of Iran. J Parasit Dis. 2015;39(3):545-9. [PubMed ID: 26345068]. https://doi.org/10.1007/s12639-013-0393-3.
-
9.
Edvinsson B, Jalal S, Nord CE, Pedersen BS, Evengard B. DNA extraction and PCR assays for detection of Toxoplasma gondii. APMIS. 2004;112(6):342-8. [PubMed ID: 15511271]. https://doi.org/10.1111/j.1600-0463.2004.apm1120604.x.
-
10.
Ibebuike K, Mantanga L, Emereole O, Ndolo P, Kajee A, Gopal R, et al. Cerebellar toxoplasmosis in HIV/AIDS infant: case report and review of the literature. Neurol Sci. 2012;33(6):1423-8. [PubMed ID: 22286317]. https://doi.org/10.1007/s10072-012-0960-x.
-
11.
Ghasemian M, Maraghi S, Saki J, Pedram M. Determination of antibodies (IgG, IgM) against Toxoplasma gondii in patients with cancer. Iran J Parasitol. 2007;2(4):1-6.
-
12.
Fouladvand M, Barazesh A, Naeimi B, Keivan Z, Tajbakhsh S. Seroprevalence of toxoplasmosis in high school girls in Bushehr city, South west of Iran, 2009. Afr J Microbiol Res. 2010;4(11):1117-21.
-
13.
Asgari Q, Sarnevesht J, Kalantari M, Sadat SJ, Motazedian MH, Sarkari B. Molecular survey of Toxoplasma infection in sheep and goat from Fars province, Southern Iran. Trop Anim Health Prod. 2011;43(2):389-92. [PubMed ID: 20936348]. https://doi.org/10.1007/s11250-010-9704-1.
-
14.
Sarkari B, Asgari Q, Bagherian N, Ashkani Esfahani S, Kalantari M, Mohammadpour I, et al. Molecular and serological evaluation of toxoplasma gondii infection in reared turkeys in Fars Province, Iran. Jundishapur J Microbiol. 2014;7(7):11598. [PubMed ID: 25368800]. https://doi.org/10.5812/jjm.11598.
-
15.
Seifollahi Z, Sarkari B, Motazedian MH, Asgari Q, Ranjbar MJ, Abdolahi Khabisi S. Protozoan parasites of rodents and their zoonotic significance in Boyer-Ahmad district, Southwestern Iran. Vet Med Int. 2016;2016:3263868. [PubMed ID: 26998380]. https://doi.org/10.1155/2016/3263868.
-
16.
Sarkari B, Abdolahi Khabisi S. Severe congenital toxoplasmosis: a case report and strain characterization. Case Rep Infect Dis. 2015;2015:851085. [PubMed ID: 25685568]. https://doi.org/10.1155/2015/851085.
-
17.
Fuentes I, Rubio JM, Ramirez C, Alvar J. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol. 2001;39(4):1566-70. [PubMed ID: 11283088]. https://doi.org/10.1128/JCM.39.4.1566-1570.2001.
-
18.
Khan A, Su C, German M, Storch GA, Clifford DB, Sibley LD. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J Clin Microbiol. 2005;43(12):5881-7. [PubMed ID: 16333071]. https://doi.org/10.1128/JCM.43.12.5881-5887.2005.
-
19.
Ajzenberg D, Cogne N, Paris L, Bessieres MH, Thulliez P, Filisetti D, et al. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186(5):684-9. [PubMed ID: 12195356]. https://doi.org/10.1086/342663.