Abstract
Background:
In recent decades, multidrug-resistant non-fermenting Gram-negative pathogens, particularly Acinetobacter baumannii and Pseudomonas aeruginosa, have been recognized as a major cause of healthcare-associated and nosocomial infections and outbreaks.Objectives:
The aim of this study was to determine the prevalence and pattern of antibiotic resistance in A. baumannii and P. aeruginosa isolates collected from intensive care units (ICUs).Methods:
One hundred fifty-five clinical isolates, including 80 (51.6%) isolates of A. baumannii and 75 (48.4%) isolates of P. aeruginosa, from hospitalized patients in the ICUs of a teaching hospital in Ahvaz, Iran, were collected from January 1 to December 30, 2013. The organisms were identified with conventional bacteriological methods, and antimicrobial susceptibility testing was performed on all isolates in accordance with clinical laboratory and standards institute (CLSI) guidelines.Results:
The maximum resistance rates among A. baumannii isolates were observed for ciprofloxacin and trimethoprim-sulfamethoxazole (96.9% and 95.2%, respectively). For P. aeruginosa isolates, the maximum resistance rates were reported for ceftriaxone and trimethoprim-sulfamethoxazole (97.2% and 92.4%, respectively).Conclusions:
The majority of A. baumannii and P. aeruginosa isolates were found to be resistant to commonly recommended antibiotics. Therefore, surveillance of antibiotic consumption and proper antibiotic administration guidelines are essential for preventing major outbreaks in the future.Keywords
Resistance Susceptibility Intensive Care Unit Acinetobacter baumannii Pseudomonas aeruginosa
1. Background
Non-fermenting Gram-negative bacterial pathogens (NFGNBPs) are a common cause of opportunistic infections in critically ill or immunocompromised patients (1). NFGNBPs are commonly responsible for infections in intensive care units (ICUs) (2), and they have been associated with prolonged hospitalization, considerable costs, and notable mortality and morbidity (3, 4).
The treatment of Gram-negative organisms represents a great challenge for the healthcare community (2, 3), mainly due to the significant increase in resistance to antimicrobial agents. Antimicrobial resistance among Gram-negative pathogens is a growing problem and a global concern (2, 5), as traveling and trading permits the spread of resistant pathogens among countries and continents (5). Important NFGNBPs in the United States are Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia, and Burkholderia cepacia (1). Two multidrug-resistant (MDR) non-fermenting Gram-negative pathogens of great importance presently are A. baumannii and P. aeruginosa (1, 6).
In recent decades, A. baumannii has been recognized as a leading nosocomial pathogen, particularly among patients admitted to the ICU (6, 7). In the past, most A. baumannii isolates were sensitive to carbapenems (7), but in the last two decades, due to the widespread use of these antibiotics, MDR and extensively drug-resistant (XDR) strains of A. baumannii have been increasingly reported and have emerged as a critical problem worldwide (8, 9). Members of the genus Acinetobacter have been implicated as the cause of many serious infectious diseases, such as respiratory infections, bacteremia, urinary tract infections, meningitis, and endocarditis, mostly engaging critically ill or immunocompromised patients (1, 8). The incidence of A. baumannii infections has increased over the last few decades (10) and this has been recognized as the third most common causative agent of infections in ICU patients, with mortality rates of 26% - 68% (7). The outcomes of patients with MDR or XDR A. baumannii infections seem to be worse (10).
Pseudomonas aeruginosa is considered one of the major causes of opportunistic infections (6) and is recognized as a common cause of morbidity and mortality among hospitalized patients in the United States (1). Pseudomonas aeruginosa is recognized as the main cause of a wide range of healthcare-associated infections, especially in immunocompromised patients and those with cystic fibrosis (6). This bacterium is distinguished by a high intrinsic resistance to antibiotics; it can also acquire resistance to the antibiotics used, and therefore its antibacterial resistance rate continues to increase (1).
2. Objectives
The aim of this study was to assess the prevalence and pattern of antibiotic resistance in A. baumannii and P. aeruginosa isolates collected from ICUs at Imam Khomeini hospital in Ahvaz, Iran, in order to provide essential information for a more rational use of broad-spectrum antibiotics and to justify infection-control protocols in order to restrict the spread of MDR bacteria and NFGNBPs.
3. Methods
3.1. Bacterial Isolates
This study was conducted in ICUs at Imam Khomeini hospital, a teaching hospital associated with Ahvaz Jundishapur University of Medical Sciences, from January 1 to December 30, 2013. During this time, a total of 2.174 patients were admitted to the various ICUs of this hospital, and 552 clinical specimens were collected from these patients. The ICUs included the surgical ICU, the internal medicine ICU, the cardiovascular ICU, the coronary care unit (CCU), and the neonatal intensive care unit (NICU). Patients who had taken prophylactic antibiotics were excluded from the study.
The isolates were obtained from a wide range of clinical specimens, including blood, pleural fluid, urine, tracheal tube discharge, secretions, wounds, abscesses, eyes (cornea, conjunctivae, foreign bodies, lenses, etc.), surgical wounds, cerebrospinal fluid, ascites, pericardial fluid, and articular fluids. The clinical specimens were obtained in accordance with the standard guidelines (11).
Laboratory tests for different specimens were performed as follows: blood and eosin-methylene blue (EBM) agar plates were used for urine specimens; trypticase soy broth (TSB) followed by sub-culturing for blood and MacConkey agar plates were used for blood specimens; and blood, MacConkey agar plates, and sodium thioglycollate broth were used for other specimens. Each specimen was incubated at 35°C for 24 - 48 hours on its specific agar plate in order to boost bacterial growth (11).
The agar plates used in this study were obtained from Merck KGaA (Darmstadt, Germany) and Laboratorios Conda (Canada). Quality control was performed with Escherichia coli ATCC 25922, Staphylococcus ATCC 25923, P. aeruginosa ATCC 27853, and Enterococci ATCC 29212. All of the suspected colonies were evaluated with Gram-staining, colonial morphology, oxidation-fermentation testing, ortho-Nitrophenyl-β-galactoside (ONPG) testing, and other biochemical reactions (11, 12). The suspected colonies were identified as A. baumannii if they were oxidase- and nitrate-negative, while having dextrose-positive reactions and growing at 44°C (11).
3.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility of the isolated organisms was evaluated by the disk diffusion method in accordance with clinical laboratory and standards institute (CLSI) guidelines (13). Concisely, a suspension of each isolate was assembled so that the turbidity was equal to 0.5 McFarland standards, and was then cultivated onto Mueller-Hinton agar. After 18 24 hours of incubation at 35°C, the inhibition zone diameter was measured, the collected data were compared to CLSI standards, and each isolate was eventually reported as susceptible, intermediate, or resistant (13). The antibiotic disks used in this study were obtained from Padtan Teb (Iran) and ROSCO (Denmark).
An Acinetobacter isolate was defined as MDR if it was resistant to all generations of cephalosporins, fluoroquinolones, and aminoglycosides. XDR Acinetobacter was defined as non-susceptibility to cephalosporins, fluoroquinolones, aminoglycosides, and carbapenems (14).
3.3. Data Management and Statistical Analysis
The data for antimicrobial susceptibility testing were managed and converted into a standard format using WHONET software (version 5.6, WHO, Switzerland) and were statistically analyzed with SPSS software (version 19, SPSS Inc., Chicago, IL, USA). A two-tailed P value of < 0.05 was considered statistically significant. This study was approved by the ethics committee of the school of medicine, Ahvaz Jundishapur University of Medical Sciences.
4. Results
A total of 155 clinical isolates of NFGNBPs, including 80 (51.6%) isolates of A. baumannii and 75 (48.4%) isolates of P. aeruginosa, were obtained from patients hospitalized in the ICUs of Imam Khomeini Hospital in Ahvaz, Iran. A. baumannii and P. aeruginosa were most frequently isolated from the surgical ICU (18.06%) and the internal medicine ICU (25.80%), respectively (Table 1). The most common source of Acinetobacter and P. aeruginosa isolates was tracheal secretions (47.5%, and 52%, respectively). Figure 1 shows the distribution of isolates from different specimen sites.
Prevalence of Each Organism Among 155 Clinical Specimens Isolated from Different ICU Wards
| Organism | Prevalence of Isolates among Specimens from all ICU Wards, % | Prevalence of Each Organism in Different Wards, % | ||||
|---|---|---|---|---|---|---|
| CCU | CVICU | IMICU | SICU | NICU | ||
| Acinetobacter baumannii | 51.6 | 0.64 | 6.45 | 16.77 | 18.06 | 9.67 |
| Pseudomonas aeruginosa | 48.4 | 0 | 3.22 | 25.80 | 14.19 | 5.16 |
Distribution of Isolates in Different Specimen Sites
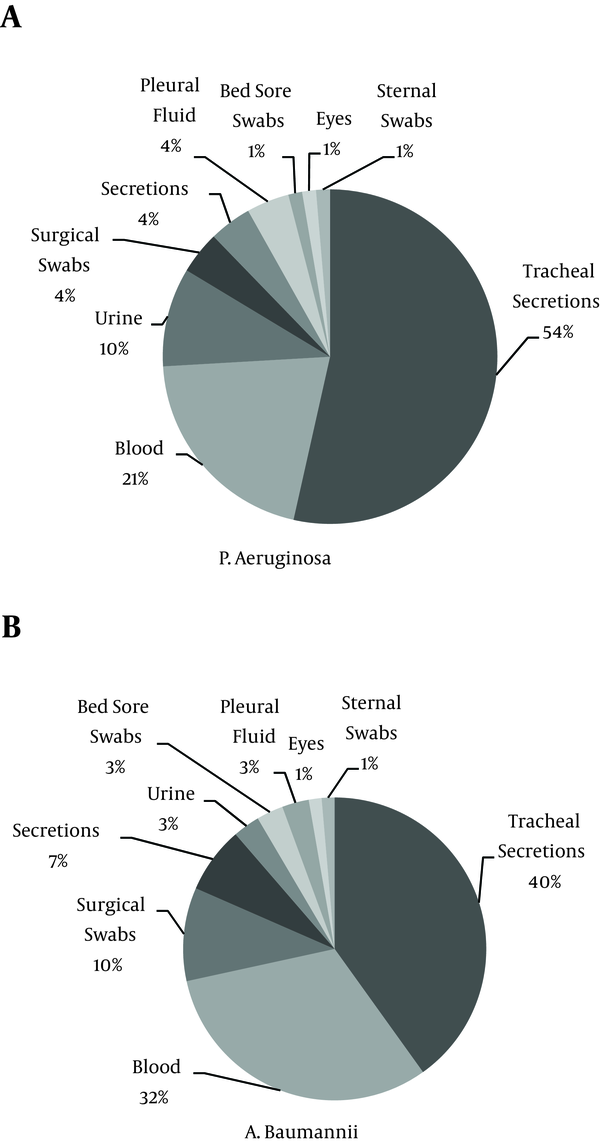
The maximum Acinetobacter resistance rates were observed for ciprofloxacin and trimethoprim-sulfamethoxazole (96.9% and 95.2%, respectively). The results of antibiotic susceptibility testing for Acinetobacter isolates are shown in Table 2. Among 80 Acinetobacter isolates, 65 were XDR (81.3%), one was MDR (1.2%), and 14 were susceptible (17.5%) to the antimicrobial drugs most commonly used in ICU settings.
Results for Antibiotic Susceptibility Testing of 80 A. baumannii Isolates
| Antibiotic | Resistant, % | Intermediate, % | Susceptible, % |
|---|---|---|---|
| Amikacin | 84.8 | 0 | 15.2 |
| Cefepime | 84.7 | 5.6 | 9.7 |
| Ceftriaxone | 92.9 | 6.5 | 1.3 |
| Ciprofloxacin | 96.9 | 0 | 3.1 |
| Tetracycline | 81.8 | 18.2 | 0 |
| Trimethoprim-sulfamethoxazole | 95.2 | 0 | 4.8 |
| Meropenem | 88.5 | 0 | 11.5 |
| Ampicillin-sulbactam | 42.4 | 18.6 | 39 |
The maximum P. aeruginosa resistance rates were reported to ceftriaxone and trimethoprim-sulfamethoxazole (97.2% and 92.4% respectively). The results of antibiotic susceptibility testing of P. aeruginosa isolates are shown in Table 3. There was no statistically significant difference in antimicrobial resistance between the genders, but a statistical analysis showed a significant difference in antimicrobial resistance among different ICU wards. The NICU had a remarkably lower resistance to three antibacterial agents (amikacin, cefepime, and meropenem; P < 0.0001) in comparison to the other ICUs.
Results of Antibiotic Susceptibility Testing of 75 P. aeruginosa Isolates
| Antibiotic | Resistant, % | Intermediate, % | Susceptible, % |
|---|---|---|---|
| Amikacin | 73.5 | 4.4 | 22.1 |
| Cefepime | 77.6 | 6.9 | 15.5 |
| Ceftriaxone | 97.2 | 1.4 | 1.4 |
| Ciprofloxacin | 68.2 | 6.1 | 25.8 |
| Gentamicin | 85.7 | 0 | 14.3 |
| Piperacillin | 57.1 | 0 | 42.9 |
| Piperacillin-tazobactam | 46.9 | 0 | 53.1 |
| Tetracycline | 50 | 16.7 | 33.3 |
| Trimethoprim-sulfamethoxazole | 92.4 | 1.5 | 6.1 |
| Meropenem | 64.3 | 4.3 | 31.4 |
| Ceftazidime | 71.2 | 7.6 | 21.2 |
5. Discussion
Infections in ICUs are a formidable healthcare concern (15), in particular the MDR A. baumannii and P. aeruginosainfections that have become increasingly common over the last few decades, especially among critically ill or immunocompromised patients (16). This resistance to common antibiotic agents may lead to outbreaks that can be very challenging for healthcare personnel to control.
There have been relatively few studies on antibiotic resistance patterns of NFGNBPs in ICUs in Iran. In 2009, Vahdani et al. (10) reported an A. baumannii resistance rate of 85% to ciprofloxacin and co-trimoxazole and a 58% resistance rate to amikacin in Tehran. The present study revealed a higher resistance to these antibiotics (96.9% for ciprofloxacin, 95.2% for co-trimoxazole, and 84.8% for amikacin).
The results of a study that Shoja et al. (7) carried out on the ICUs of two hospitals in the city of Ahvaz from 2010 to 2012 showed that the resistance rate of A. baumannii to meropenem was 96.1%. The findings of the present study indicate a marked reduction in this rate, to 88.5%. In comparison to Shoja et al.’s study, our results also showed lower resistance rates to ceftriaxone (92.9% versus 96.1%), cefepime (84.7% versus 96.1%), and ampicillin-sulbactam (42.4% versus 69.4%), as well as lower susceptibility to tetracycline (0% versus 7.3%), and trimethoprim-sulfamethoxazole (4.8% versus 7.8%). This marked difference in Acinetobacter resistance might be due to the fact that Shoja et al. did not include NICU specimens, as we did in the present study.
In 2013, Nasrolahei et al. (8) evaluated 100 A. baumannii isolates from burn patients and ICU patients in Tehran and Sari, and reported that 37.1% of A. baumannii isolates were XDR. The present study revealed a markedly higher rate of XDR A. baumannii isolates (81.3%). In 2013, Salehi et al. (17) conducted a study on the antimicrobial resistance pattern of clinical isolates of P. aeruginosa from patients hospitalized in trauma and burn ICUs in Tehran; they reported resistance rates of 82.2% for ceftriaxone, 44.9% for piperacillin, and 48.03% for tetracycline. The present study revealed substantially higher resistance rates to these antibiotics (97.2%, 57.1%, and 50%, respectively). This analysis revealed that there has been an overall increase in the antibiotic resistance pattern of P. aeruginosa.
In 2011, Sader et al. reviewed the antimicrobial susceptibility patterns of 5,989 Gram-negative bacterial isolates from ICU patients in hospitals in the United States and Europe (3). The results for A. baumannii isolates in the United States revealed susceptibility rates of 51.8% to amikacin, 42.8% to ampicillin-sulbactam, 33.7% to cefepime, 34.3% to ciprofloxacin, and 42.8% to meropenem. The results for A. baumannii isolates in Europe showed susceptibility rates of 51.1% to amikacin, 30.2% to ciprofloxacin, and 43.2% to meropenem; these were all shown to be remarkably lower in the present study (Table 2 shows the susceptibility rates to different antibiotic agents among A. baumannii isolates). The results for P. aeruginosa isolates in the United States showed susceptibility rates of 94.8% to amikacin, 91.4% to cefepime, 84.6% to ceftriaxone, 87.3% to ciprofloxacin, 92.7% to gentamicin, 85.9% to piperacillin-tazobactam, and 95.4% to meropenem. The results for P. aeruginosa isolates in Europe showed susceptibility rates of 90.5% to amikacin, 76.7% to cefepime, 73.7% to ceftriaxone, 76.3% to ciprofloxacin, 83.3% to gentamicin, 71.4% to piperacillin-tazobactam, and 95.8% to meropenem. The susceptibility rates for P. aeruginosa were significantly lower in the present study (Table 3).
To our knowledge, there are few studies investigating the relationship of independent factors with NFGNBP resistance rates. In this study, we evaluated NFGNBP resistance rates according to the patients’ genders, and found no statistically significant differences. However, the results of a study conducted by Xu et al. (18) in China to investigate the antimicrobial resistance of A. baumannii showed a higher antimicrobial resistance to five antibiotics in males.
We also analyzed the NFGNBP resistance rate according to different ICU wards. Statistically significant differences in NFGNBP resistance were reported among three drugs (amikacin, cefepime, and meropenem; P < 0.0001). It is noteworthy that all of the NFGNBP resistance rates were significantly lower in the NICU. This might be due to different types of bacteria isolated in the NICU compared to other ICUs, which is attributed to different antibiotic policies and different antibacterial resistance patterns. Ariffan et al. (19) also reported a lower antibacterial resistance in NICUs compared to adult ICUs.
The present study revealed a high number of A. baumannii and P. aeruginosa strains with resistance to virtually all antibiotics tested. The marked increase in the number of XDR A. baumannii strains and the significant reduction in susceptibility patterns for P. aeruginosa emphasize the evolving need for a more rational use of broad-spectrum antibiotics, in addition to an immediate reconsideration of infection-control protocols, in order to restrict the spread of these pathogens.
It should be noted that our data were obtained from only one hospital, so the continuous supervision of antimicrobial resistance of NFGNBPs in Ahvaz is an evolving necessity in order to produce adequate representative data. Further studies are recommended, focusing on various independent factors affecting antibacterial resistance, including age, gender, and different hospital wards.
Acknowledgements
References
-
1.
McGowan JEJ. Resistance in nonfermenting gram-negative bacteria: multidrug resistance to the maximum. Am J Infect Control. 2006;34(5 Suppl 1):29-37. [PubMed ID: 16813979]. https://doi.org/10.1016/j.ajic.2006.05.226.
-
2.
Rahal JJ. Antimicrobial resistance among and therapeutic options against gram-negative pathogens. Clin Infect Dis. 2009;49 Suppl 1:4-10. [PubMed ID: 19619021]. https://doi.org/10.1086/599810.
-
3.
Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis. 2014;78(4):443-8. [PubMed ID: 24492025]. https://doi.org/10.1016/j.diagmicrobio.2013.11.025.
-
4.
Vakili B, Fazeli H, Shoaei P, Yaran M, Ataei B, Khorvash F, et al. Detection of colistin sensitivity in clinical isolates of Acinetobacter baumannii in Iran. J Res Med Sci. 2014;19(Suppl 1):67-70. [PubMed ID: 25002899].
-
5.
Kang CI, Song JH. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infect Chemother. 2013;45(1):22-31. [PubMed ID: 24265947]. https://doi.org/10.3947/ic.2013.45.1.22.
-
6.
Sanchez A, Gattarello S, Rello J. New treatment options for infections caused by multiresistant strains of Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli. Semin Respir Crit Care Med. 2011;32(2):151-8. [PubMed ID: 21506051]. https://doi.org/10.1055/s-0031-1275527.
-
7.
Shoja S, Moosavian M, Peymani A, Tabatabaiefar MA, Rostami S, Ebrahimi N. Genotyping of carbapenem resistant Acinetobacter baumannii isolated from tracheal tube discharge of hospitalized patients in intensive care units, Ahvaz, Iran. Iran J Microbiol. 2013;5(4):315-22. [PubMed ID: 25848498].
-
8.
Nasrolahei M, Zahedi B, Bahador A, Saghi H, Kholdi S, Jalalvand N, et al. Distribution of bla(OXA-23), ISAba , Aminoglycosides resistant genes among burned & ICU patients in Tehran and Sari, Iran. Ann Clin Microbiol Antimicrob. 2014;13:38. [PubMed ID: 25252850]. https://doi.org/10.1186/s12941-014-0038-0.
-
9.
Zhao SY, Jiang DY, Xu PC, Zhang YK, Shi HF, Cao HL, et al. An investigation of drug-resistant Acinetobacter baumannii infections in a comprehensive hospital of East China. Ann Clin Microbiol Antimicrob. 2015;14:7. [PubMed ID: 25643932]. https://doi.org/10.1186/s12941-015-0066-4.
-
10.
Vahdani P, Yaghoubi T, Aminzadeh Z. Hospital acquired antibiotic-resistant acinetobacter baumannii infections in a 400-bed hospital in Tehran, Iran. Int J Prev Med. 2011;2(3):127-30. [PubMed ID: 21811653].
-
11.
Mahon CR, Lehman DC, Manuselis G. Textbook of Diagnostic Microbiology. 5 ed. Houston: Elsivier; 2014.
-
12.
Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott's Diagnostic Microbiology. 10 ed. St. Louis: Mosby; 1998.
-
13.
Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline. Wayne: Clinical and Laboratory Standards Institute; 2005.
-
14.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-81. [PubMed ID: 21793988]. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
-
15.
Tukenmez Tigen E, Dogru A, Koltka EN, Unlu C, Gura M. Device-associated nosocomial infection rates and distribution of antimicrobial resistance in a medical-surgical intensive care unit in Turkey. Jpn J Infect Dis. 2014;67(1):5-8. [PubMed ID: 24451094].
-
16.
Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010;30(5):364-9. [PubMed ID: 20697174]. https://doi.org/10.4103/0256-4947.67073.
-
17.
Salehi M, Hekmatdoost M, Hosseini F. Quinolone resistance associated with efllux pumps mexAB-oprM in clinical isolates of Pseudomonas aeruginosa. J Microb World. 2014;6(4):290-8.
-
18.
Xu T, Xia W, Rong G, Pan S, Huang P, Gu B. A 4-year surveillance of antimicrobial resistance patterns of Acinetobacter baumanni in a university-affiliated hospital in China. J Thorac Dis. 2013;5(4):506-12. [PubMed ID: 23991309]. https://doi.org/10.3978/j.issn.2072-1439.2013.08.36.
-
19.
Ariffin N, Hasan H, Ramli N, Ibrahim NR, Taib F, Rahman AA, et al. Comparison of antimicrobial resistance in neonatal and adult intensive care units in a tertiary teaching hospital. Am J Infect Control. 2012;40(6):572-5. [PubMed ID: 22854380]. https://doi.org/10.1016/j.ajic.2012.02.032.