Abstract
Background:
The immune system provides protection against infectious diseases that are caused by various microorganisms, in particular pathogenic fungi. Utilization of herbal immunostimulants is one solution to improve the immunity of humans and to decrease their susceptibility to infectious diseases.Objectives:
The current study aimed to investigate the immunostimulatory effects of the aqueous extract of Heracleum persicum on mouse peritoneal macrophages.Materials and Methods:
The present in vitro study investigated the effect of the aqueous extract of H. perscium on the viability of macrophages and nitric oxide (NO) production using microculture tetrazolium (MTT) assay and Griess method, respectively. The effects on fungicidal activity and reactive oxygen species (ROS) production of stimulated peritoneal macrophages were also studied using killing method and nitroblue tetrazolium (NBT) assay, respectively.Results:
The aqueous extract of H. persicum (Hp-W) at concentration of 10 mg/ mL resulted in a significant increase in NO production (8.17 nmol) by macrophages (P < 0.05). Moreover, H. persicum had a stimulatory effect on the level of ROS (P < 0.05) and a strong candidacidal activity in macrophages treated with 20 mg/ mL of the extract (P < 0.05).Conclusions:
The aqueous extract of H. persicum showed a significant immunostimulatory activity on macrophages. To clarify the exact mechanisms of this activity, more studies should be done with purified immunostimulatory components of H. persicum in future.Keywords
Immunostimulatory activity Heracleum persicum MTT assay NBT assay Killing Candida albicans
1. Background
Innate immunity has a key role in preventing the host against opportunistic infections. Among various immune cells, macrophages are one of the research foci of the immunology community. Macrophages play a significant role against many opportunistic microbs especially Candida species (1). Candida albicansis an important commensal microflora on digestive tracts and mucosal barriers (2). The host immune system is the major factor balancing the transition from commensalism to pathogenicity of C.albicans (3).
Candida infections occur predominantly in patients who suffer some kinds of systemic or local immunosuppression such as persons with diabetes, patients infected with HIV, patients receiving corticosteroid or cytotoxic chemotherapy particularly for hematologic malignancies, persons exposed to prolonged antibiotic treatment and recipients of organ or stem cell transplantation (4). Reactive oxygen species (ROS) and nitric oxide (NO) are also the main mechanisms by macrophages for killing these fungal agents (5).
One of the most promising alternatives to classical antibiotic treatment is the use of immunomodulators to enhance the host defense response (6). There are several immunomodulators with botanical origins such as mushrooms, algae, lichens and higher plants. In this regard, some herbs with immunomodulartory activities including Viscum album (V. album), Withaniasomnifera and Allium sativum have been reported by different investigators (6-8). The predominant components including polysaccharides, lectins, proteins and peptides are known to stimulate the immune system (6, 9). Davis and Kuttan (7) showed that treatment with 20 mg of W. somnifera root extract resulted in an enhancement in phagocytic activity of peritoneal macrophages. In a study by Choi et al. (10), the methanolic extract of Caloplaca ragalis (CR-ME) increased the production of tumor necrosis factor-α (TNF- α) and NO by peritoneal macrophages. However, CR-ME had a little effect on the levels of ROS.
H. persicum, commonly known as Golpar in Persian, is a flowering plant in the Apiaceae family that grows wild in humid alpine regions of Iran (11, 12). This aromatic plant is used as a flavoring ingredient in most of Iranian food products. Based on Iranian traditional medicine, it is used to relieve flatulence and stomach aches. It is also used to disinfect the stomach, antioxidants, and cure a poor appetite (13, 14). Preliminary phytochemical analysis of H. persicum extract showed the presence of alkaloids, terpenoids, triterpenes and steroids (15).
2. Objectives
The current study was undertaken to estimate the in vitro stimulatory effects of aqueous extract of H. persicum on viability, NO and ROS productions of peritoneal macrophages and candidacidal activity.
3. Materials and Methods
3.1. Plant Collection and Identification
The aerial parts of H. persicum were harvested from Khorasan province, northeast of Iran, in 2007. Botanical identification was performed at the Herbarium of Pharmacognosy Department, School of Pharmacy, Shaheed Beheshti University of Medical sciences, Iran. The voucher botanic specimen was 1312.
3.2. Preparation of Extraction
The plant seeds were ground into fine powder. For aqueous extract preparation, 100 g of plant powder was mixed with 400 mL of water, boiled for 10 min and filtered by Whatman paper (No. 1). The resulting solution was frozen and lyophilized for 96 h at – 50°C and 0.04 mbar (Snijder scientific Ltd, Holland). The residue was coded with letter (Hp-W).
3.3. Candida albicans Strain
Candida albicans (ATCC 10231) was cultured on Sabouraud glucose agar (Merck Co., Darmstadt, Germany) at 35°C for 3 days, harvested and kept at 4°C until used.
3.4. Animals and Peritoneal Macrophages Preparation
Male Balb/c mice (6 to 8 weeks of age, weighting 18-25 g) were purchased from the Animal Breeding Laboratory of the Faculty of Medicine, Shahed University, Tehran, Iran. All animals were housed and handled according to institutionally recommended guidelines.
The animals were sacrificed and peritoneal exudates cells were harvested by lavage using 5 mL of cold PBS (5 mg/mL, pH 7.2) and poured in sterile plastic tubes. Cells were pooled, resuspended in RPMI 1640 supplemented with 5% FBS (GIBCO, Grand Island, NY, USA) and cultured in 96-well flat-bottom microtiter plates at a final concentration of 4×105 cells per well. After 2 h, the debris and non-adherent cells were removed from the wells. Then, the monolayer macrophages were reincubated at 37°C for 20 h along with different concentrations of extract (5, 10 and 20 mg/mL).
3.5. Macrophages Viability Assay
MTT {3- (4, 5-dimethylthiaozle-2-yl) -2, 5 – diphenyle – tetra zolium bromide} powder (Merck Co., Darmstadt, Germany) was dissolved in PBS (5 mg/ mL, pH 7.4), filtered and stored at -20°C until used. The MTT assay was performed in the 96-well plates (16). Briefly, the wells were washed three times with complete medium, then 180-µl aliquots of medium and 20- µl aliquots of MTT solution (5 mg/ mL of PBS) were added to each well at the established time. After 2 h of incubation at 37°C and 5% CO2 for exponentially growing cells and 15 min for steady-state confluent cells, the media were removed and formazan crystals were solubilized with 175 µl of DMSO. The plates were then read on a Microplate reader Model 450 (Bio-Rad Laboratories, Hercules, CA, USA) at 540-nm wave length.
3.6. Extracellular NO Production
NO released into the supernatants of mouse macrophages was determined by the standard Griess reaction by adding 50 μl of test solution to 96-well flat-bottomed plates containing 50 μl of Griess reagent [1% sulfanilamide/0.1% N-(1-naphthyl) ethylenediamine dihydrochloride/2.5% H3PO4]. The samples were assayed in quadruplicate. After 15 min at room temperature, the absorbance of each well was measured in a Multiskan MS microplate reader (Labsystems Oy, Helsink, Finland) at 540 nm and the nitrite concentration was determined from a standard curve of sodium nitrite (17).
3.7. Intracellular ROS Production
Determination of intracellular ROS production by macrophages was determined using NBT assay by Gentle and Thompson method (18). Briefly, peritoneal macrophages were seeded at a density of 1×105 cells per well and treated with aqueous extract of H. persicum (Hp-W, 20 mg/mL) with or without N-formyl-methionyl-leucyl-phenylalanine plus lipopolysaccharide (f MLP+LPS) stimulator for 20 h in 96-well flat-bottom microtiter plate. Then, 50% of RPMI and 50% of NBT sterile solution (0.1%) were added to each well and incubated at 37°C for 1 h. The supernatant was removed, and 50 mL of pyridine was added, and absorbances were read using ELISA reader at 540 nm. Binding of macrophage receptors with f MLP and LPS resulted in hydrolysis of phosphatidylinositol diphosphate by a specific phospholipase C, subsequently leading to a rise in inositol trisphosphate, secretion of lysosomal enzymes, NADPH oxidase and ROS production (19).
3.8. Candidacidal Activity of Macrophages
Candida albicans ATCC 10231 was used in the fungicidal assay as a target microorganism. Macrophages (1×106 cell/mL) were pre-incubated with Hp-W for 3 h, and then the supernatant was replaced with fresh medium. Macrophages and C. albicans were mixed at 1:1 ratio and incubated under constant rotation at 39.5°C and 5% CO2. After 1 h, 50 mL of the suspension were added to 4.95 mL of chilled water and thoroughly mixed. Subsequently, 50 mL from this suspension was distributed on a Petri dish containing Sabouraud glucose agar (Merck Co., Darmstadt, Germany) and incubated at 37°C for 48 h. The colonies were counted and data were illustrated as follow (3):
Fungicidal activity = [1 - CFU experimental culture/CFU untreated] × 100
3.9. Statistical Analysis
Data were analyzed using a one-way analysis of variances (ANOVA) and presented as Mean + SEM. The P values < 0.05 were considered as significant differences (SPSS version 10).
4. Results
4.1. Macrophages Viability
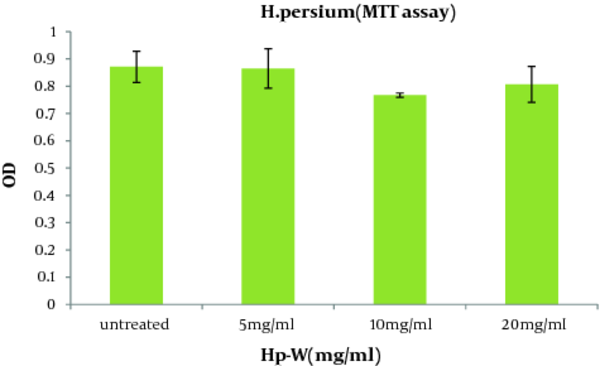
Regarding the effect of the aqueous extract of H. persicum (Hp-W) on viability of macrophages, there were no significant differences between Hp-W and control group at the applied doses ( Figure 1 ).
The Viability of the Peritoneal Macrophages Treated with the Aqueous Extract of H. persicum (Hp-W) for 16 h (Mean ± SEM).
4.2. NO Production
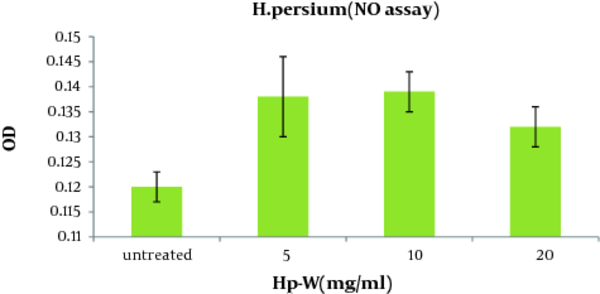
As shown in Figure 2 , the aqueous extract of H. persicum (Hp-W) at the concentration of 10 mg/ mL induced a significant increase in NO production when compared to control group (P < 0.05). The concentrations of NO production in control group and macrophages treated with Hp-W (10 mg/mL) were 2.89 and 8.17 nmol, respectively.
NO Production of Peritoneal Macrophages Stimulated With the Aqueous Extract of H. persicum (Hp-W) for 16 h (Mean ± SEM).
4.3. ROS Production
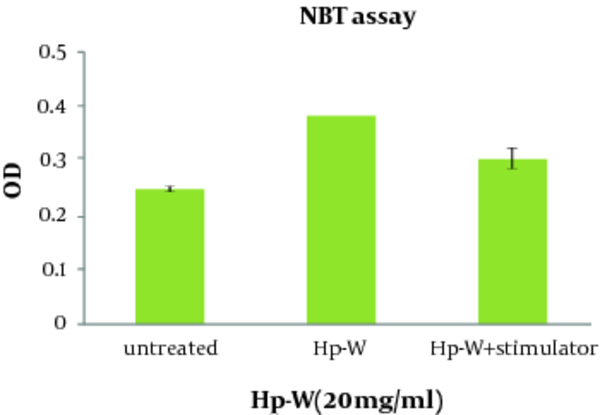
The effect of Hp-W on ROS production was given in Figure 3 . Increasing ROS production was found significant by Hp-W at the concentration of 20 mg/mL (P < 0.05). In addition, combination of Hp-W at 20 mg/ mL with f MLP + LPS significantly stimulated ROS production compared to the control group (P < 0.05), but there was no statistically significant difference between Hp-W and f MLP + LPS, which means that f MLP and LPS cannot increase ROS production.
ROS Production of Peritoneal Macrophages Stimulated With the Aqueous Extract of H. persicum (Hp-W) and Hp-W + Stimulators (LPS+f MLP) for 16 h (Mean ± SEM).
4.4. Killing of C. albicans by Macrophages
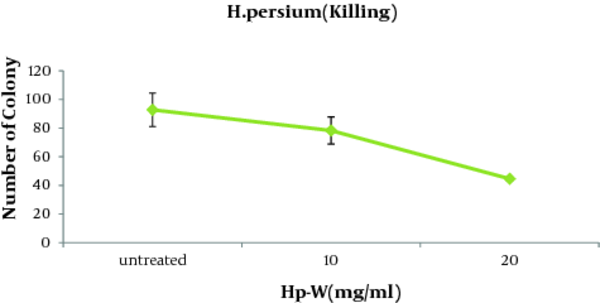
In order to evaluate the possible effect of the aqueous extract on the fungicidal activity of macrophages against C. albicans, fungicidal activity test was performed. As illustrated in Table 1, candidacidal activity of macrophages augmented significantly at the presence of Hp-W extract. According to Figure 4 , killing of the macrophages (51.9%) were significantly stimulated with Hp-W extract (lower number of Candida colonies were obtained) at the concentration of 20 mg/mL when compared to control group (P < 0.05).
Fungicidal Activity of Peritoneal Macrophages Treated With the Aqueous Extract of H. persicum (Hp-W) in Different Concentrations and Challenged with C. albicans.
Number of Colonies After Co-Culture of the Aqueous Extract of H. persicum (Hp-W) Treated and Untreated (Control) Macrophages in Order to Evaluate the Fungicidal Activity Against C. albicans.
5. Discussion
The study of host resistance against systemic fungal infections has received considerable attention in the past decade (16). Until that time, most immunological investigations of these organisms concerned with the isolation and characterization of antigens for use in vaccines, diagnoses and epidemiological studies. The lack of information about the role of local defenses, antibody production and cell-mediated reactions has prompted investigations into host response to various fungi. Recent studies have pointed out the importance of innate immunity in fungal infections (17, 20).
Macrophages have an important role in the initial responses to infection before action of humoral and cellular immunity (3, 21). The function of macrophages includes phagocytosis, antigen processing and presentation, cytokine secretion and antibody dependent cell-mediated cytotoxicity (4, 5). It is approved that macrophage phagocytosis is a key to prevent Candida species invasion. These cells can damage and kill different shapes of C. albicans (yeast and hyphae) by oxidative and non-oxidative productions. Based on the literature review, there are few studies on the potential effects of herbal extracts on innate immunity (22, 23).
The present study evaluated the effect of native herbal extract of H. persicum on different activities of macrophages. The results showed no significant difference between the effects of aqueous extract (Hp-W) and control on viability of macrophages at the applied concentrations. The aqueous extract of H. persicum (Hp-W) at concentration of 10 mg/ mL induced a significant increase in NO production compared to the control group. Application of Hp-W extract at 20 mg/mL significantly increased ROS production as well. These activities could be due to the presence of flavonoids and coumarins, which can augment the macrophage responses (24). As far as we know, little information has been reported on immunostimulatory effect of H. persicum, although there are similar works on various herbs. In a study conducted by Sharififar et al. (25), H. persicum showed a stimulatory effect on both humoral and cellular immune functions in mice.
The H. persicum extract elicited a significant increase (P < 0.05) in the delayed type hypersensitivity response at doses of 100 and 200 mg/kg. There are some studies which have confirmed the immunostimulatory effects of the two other species of H. maximum and H. nepalense (26, 27). The extract of H. maximum stimulates the production of IL-6 which its production is a well-established and reliable marker of macrophage activation (27). It has been shown that the methanolic extract of H. nepalense, at a dose of 1000 mg/kg, results in a four-fold increase in haemaglutinin titer when compared to control group (26).
More specifically, C. albicansblastoconida have shown to be susceptible to the oxygen-dependent killing mechanisms of mononuclear phagocytic cells. The candidacidal activity of mononuclear phagocytic cells has been associated with the production of superoxide anion, one of the products of reactive oxygen species (ROS) metabolism which is essential for macrophages "oxidative killing" (5). Interestingly, with respect to ROS production, there was no significant difference between Hp-W and combination of stimulators with Hp-W. The exact mechanisms underlying this event remains unclear, but it seems that oral exposure of Hp-W at concentration of 20 mg/mL will be able to induce ROS production with the same strength that mitogens such as f MLP and LPS do, through different signal transduction pathways (28). Therefore, as f MLP and LPSare known as strong macrophage stimulators, Hp-W is also a suitable activator and can be included in macrophage stimulator agents.
The present study demonstrated that Hp-W at the concentration of 20 mg/ mL significantly increased candidacidal activity of macrophage compared to control group. Naeini et al. (29) showed anti-C.albicans activity of essential oil of H. persicum. Static and lethal effects of the above oil against C. albicans were 1.1 mg/ mL, representing moderate efficacy against this Candida species. The valuable effects of H. persicum have been reported. In a study by Souri et al. (30), the antioxidant activity of some furanocoumarins isolated from H. persicum was demonstrated. According to the results, antioxidant activity of crude ethyl acetate extract was stronger than that of isolated from single component. Sayyah (15) exhibited the anticonvulsant activity of acetonic extract of the seeds of H. persicum against pentylenetetrazole (PTZ) and maximal electroshock (MES)-induced seizures in mice. The extract showed a dose-dependent protective effect in both seizure models. The observed pharmacological effects could be due to alkaloids, terpenoids, and triterpenes present in the plant (15).
Finally, H. persicum can be used to enhance innate immune functions, in particular macrophage activity. Since H. persicum plant has been used as food additive from ancient time up to now, so the Hp-W can be administrated orally without any known side effects. The role of different components of H. persicum extract on the macrophage function is not fully understood. So, further studies on the effects of the components on immune cells are required and this study should be continued to establish the extract for using these components in the exact patients.
Acknowledgements
References
-
1.
Ebtekar M, Yaraee R, Ahmadiani A, Sabahi F. Kinetics of nitric oxide production and MTT reduction by HSV-1 infected macrophages. Iran J Med Sci. 2006;31(1):9-13.
-
2.
Khan ZU, Chandy R, Metwali KE. Candida albicans strain carriage in patients and nursing staff of an intensive care unit: a study of morphotypes and resistotypes. Mycoses. 2003;46(11-12):479-86. [PubMed ID: 14641621].
-
3.
Loyola W, Gaziri DA, Gaziri LC, Felipe I. Concanavalin A enhances phagocytosis and killing of Candida albicans by mice peritoneal neutrophils and macrophages. FEMS Immunol Med Microbiol. 2002;33(3):201-8. [PubMed ID: 12110482].
-
4.
Tavanti A, Campa D, Bertozzi A, Pardini G, Naglik JR, Barale R, et al. Candida albicans isolates with different genomic backgrounds display a differential response to macrophage infection. Microbes Infect. 2006;8(3):791-800. [PubMed ID: 16473540]. https://doi.org/10.1016/j.micinf.2005.09.016.
-
5.
Vazquez-Torres A, Balish E. Macrophages in resistance to candidiasis. Microbiol Mol Biol Rev. 1997;61(2):170-92. [PubMed ID: 9184009].
-
6.
Ghazanfari T, Hassan ZM, Ebrahimi M. Immunomodulatory activity of a protein isolated from garlic extract on delayed type hypersensitivity. Int Immunopharmacol. 2002;2(11):1541-9. [PubMed ID: 12433055].
-
7.
Davis L, Kuttan G. Immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2000;71(1-2):193-200. [PubMed ID: 10904163].
-
8.
Thejass P, Kuttan G. Immunomodulatory activity of Sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea). Phytomedicine. 2007;14(7-8):538-45. [PubMed ID: 17084602]. https://doi.org/10.1016/j.phymed.2006.09.013.
-
9.
Hajto T, Hostanska K, Gabius HJ. Modulatory potency of the beta-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. 1989;49(17):4803-8. [PubMed ID: 2758413].
-
10.
Choi HS, Yim JH, Lee HK, Pyo S. Immunomodulatory effects of polar lichens on the function of macrophages in vitro. Mar Biotechnol (NY). 2009;11(1):90-8. [PubMed ID: 18612687]. https://doi.org/10.1007/s10126-008-9121-x.
-
11.
Mirheidar H. Plant education. Farhang-e-Slami Press. 1996:403 p.
-
12.
Sefidkon F, Dabiri M, Mohammad N. Analysis of the oil of Heracleum persicum L.(Leaves and Flowers). J Essent Oil Res. 2002;14(4):295-7.
-
13.
Jorjani SE. Al-Aghraz al-tibbia val Mobahess al-alaiia. 1st ed. Iran: Tehran Press; 2005.
-
14.
Salehi Surmaghi H. Medicinal plants and phytotherapy. 2006. p. 55-585.
-
15.
Sayyah M, Moaied S, Kamalinejad M. Anticonvulsant activity of Heracleum persicum seed. J Ethnopharmacol. 2005;98(1-2):209-11. [PubMed ID: 15763386]. https://doi.org/10.1016/j.jep.2004.12.026.
-
16.
Klepser ME. Antifungal resistance among Candida species. Pharmacotherapy. 2001;21(8 Pt 2):124S-32S. [PubMed ID: 11501986].
-
17.
Rao YK, Fang SH, Tzeng YM. Inhibitory effects of the flavonoids isolated from Waltheria indica on the production of NO, TNF-alpha and IL-12 in activated macrophages. Biol Pharm Bull. 2005;28(5):912-5. [PubMed ID: 15863905].
-
18.
Gentle TA, Thompson RA. clinical Immunology, A practical Approach. Edited by HC Gool. 1990.
-
19.
Baggiolini M, Boulay F, Badwey JA, Curnutte JT. Activation of neutrophil leukocytes: chemoattractant receptors and respiratory burst. FASEB J. 1993;7(11):1004-10. [PubMed ID: 8396540].
-
20.
Eggimann P, Garbino J, Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect Dis. 2003;3(12):772-85. [PubMed ID: 14652203].
-
21.
Anaissie EJ, McGinnis MR, Pfaller MA. Clinical mycology with CD-ROM. 2009.
-
22.
Mediratta PK, Sharma KK, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol. 2002;80(1):15-20. [PubMed ID: 11891082].
-
23.
Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22(9):729-40. [PubMed ID: 10884593].
-
24.
Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78(2-3):133-7. [PubMed ID: 11694357].
-
25.
Sharififar F, Pournourmohammadi S, Rastegarianzadeh R, Ranjbaran O, Purhemmaty A. Immunomodulatory activity of aqueous extract of Heracleum persicum Desf. In mice. Iran J Pharm Res. 2010;8(4):287-92.
-
26.
Dash S, Nath LK, Bhise S, Kar P, Bhattacharya S. Stimulation of immune function activity by the alcoholic root extract of Heracleum nepalense D. Don. Indian J Pharm. 2006;38(5):336.
-
27.
Webster D, Taschereau P, Lee TD, Jurgens T. Immunostimulant properties of Heracleum maximum Bartr. J Ethnopharmacol. 2006;106(3):360-3. [PubMed ID: 16504434]. https://doi.org/10.1016/j.jep.2006.01.018.
-
28.
MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14(6):477-92. [PubMed ID: 11897488].
-
29.
Naeini A, Khosravi AR, Chitsaz M, Shokri H, Kamlnejad M. Anti-Candida albicans activity of some Iranian plants used in traditional medicine. J Med Mycol. 2009;19(3):168-72. https://doi.org/10.1016/j.mycmed.2009.04.004.
-
30.
Effat Souri, Hassan Farsam, Parisa Sarkheil, Fahimeh Ebadi. Antioxidant Activity of Some Furanocoumarins Isolated from Heracleum persicum. Pharmaceut Biol (Formerly International Journal of Pharmacognosy). 2004;42(6):396-399. https://doi.org/10.1080/13880200490885077.