Abstract
Background:
Malaria is the parasitic disease caused by Plasmodium infection, and is transmitted to humans through the bite of female anopheles. At present, malaria remains the most important cause of disease and death among children and adults. Approximately 214 million people are infected with malaria worldwide.Objectives:
This study aimed to analyze the distribution level and compare procalcitonin and C-reactive protein (CRP) in malaria patients before and after treatment, with focus on discussing the prognostic value of procalcitonin recovery level for recrudescence within two weeks of antimalarial treatment.Methods:
A retrospective analysis was adopted. We measured the procalcitonin and CRP inflammatory markers of 22 imported malaria patients, who were hospitalized in our hospital from January 2014 to February 2018. The trends of procalcitonin and CRP levels in three malaria patients before and after antimalarial treatment and during recrudescence were applied.Results:
The procalcitonin level was significantly elevated before antimalarial treatment in 95% (21/22) of the patients, but sharply declined within two weeks after treatment. The procalcitonin levels of 59% (13/22) and 23% (5/22) of the patients were < 0.5 ng/mL and 0.5 - 0.8 ng/mL, respectively. The difference in the procalcitonin level before and after treatment was statistically significant (Z = 4.074, P < 0.05), and the difference in the CRP level before treatment (99.63 ± 51.63 mg/L) and after treatment (20.08 ± 13.45 mg/L) was statistically significant (t = 8.167, P < 0.05). Three patients suffered from recrudescence. The procalcitonin levels of two patients were > 1.0 ng/mL and failed to recover to the normal level within two weeks after antimalarial treatment while the procalcitonin level of one patient was 0.89 ng/mL. The dynamic observation showed that the procalcitonin level progressively increased and the procalcitonin level was 0.89 ng/mL when Plasmodium was found in the blood smear.Conclusions:
The procalcitonin and CRP serum levels were significantly elevated among malaria patients. The majority of the patients recovered to the normal level within two weeks after effective antimalarial treatment and no longer suffered from recrudescence. However, the procalcitonin recovery level within two weeks after antimalarial treatment remained > 0.8 ng/mL. Hence, heightened alertness should be given to recrudescence.Keywords
1. Background
Malaria is a parasitic disease caused by Plasmodium infection, and is transmitted to humans through the bite of female anopheles. At present, malaria remains the most important cause of disease and death among children and adults. Approximately 214 million people are infected with malaria worldwide, making it one of the serious infectious diseases (1) threatening human health. There was no objective indicator for the evaluation of severity and prognosis of malaria formerly. According to a number of research bodies in recent years, procalcitonin and C-reactive protein (CRP) have relatively high values in the diagnosis and severity assessment of malaria (2-4).
Due to the induction of lipopolysaccharides and other toxic metabolites, as well as various inflammatory mediators in the systemic inflammatory response, organs and tissue cells outside the thyroid gland can produce a large amount of procalcitonin and inhibit its decomposition into calcitonin; this significantly increases the level of procalcitonin in serum. Assicot et al. (5) reported for the first time in 1993 that the serum procalcitonin content is closely related to infectious diseases, especially in the inflammatory response to bacterial infection. Later studies (6) also found that procalcitonin is related to the severity of infection and its dynamic changes are related to the prognosis of infection. Procalcitonin is also increased in neuroendocrine tumors, non-infectious systemic inflammation, trauma, parasitic infection, and other diseases (7, 8).
2. Objectives
Hence, our retrospective study was performed in malaria patients: who were treated and followed-up in our hospital. The results of this study also support the above relevant research perspectives. However, we observed that the procalcitonin level of some clinical patients still fails to recover to the normal level within two weeks after antimalarial treatment and patients suffer from recrudescence, indicating that the procalcitonin recovery level has higher clinical value for the anticipation of malaria recrudescence.
3. Methods
3.1. General Data
We recruited 22 imported malaria patients visiting Taixing People’s Hospital from January 2014 to February 2018. Eight patients were from Congo, nine patients from Angola, one patient from Pakistan, and four patients from other African countries. All patients were male, with the age range of 20 - 48 and an average age of 27.2 ± 4.8. Chills and fever were the main symptoms in the patients. All 22 patients had a fever with a body temperature of 38.1 to 41.5ºC. Moreover, 18 (81.8%) patients showed chills or shivering and 20 patients (90.9%) showed muscle ache. Eight (36.4%) patients had various degrees of gastrointestinal symptoms, including vomiting, appetite loss, and diarrhea and one (4.5%) patient suffered from renal failure.
There was no death case. The criteria for confirmed cases were (9): there is a history of epidemiology and clinical symptoms such as chills, fever, and sweating. Microscopic examination of blood smears was positive for Plasmodium or Plasmodium antigen. Before 2016, overall 10 cases were detected by blood smear microscopy alone, including nine cases of falciparum malaria and one case of ovale malaria. Since 2016, overall 12 cases, including 10 cases of falciparum malaria, one case of vivax malaria, and one case of Plasmodium vivax malaria, have been detected by blood smear microscopy and Plasmodium antigen simultaneously. Two cases of falciparum malaria and one case of ovale malaria recurred.
3.2. Study Methodology
Blood samples were collected to make thick blood membrane and thin blood membrane slices, and then hemolytic staining was carried out. Finally, the diagnosis was made based on the presence of Plasmodium parasites in the thick blood membrane under the oil lens of the microscope. Thin blood membrane was only used as the reference examination for the classification of protozoa. Malaria control professionals from the center for disease control and prevention completed the epidemiological case investigation and blood sample review within three days. Finally, the malaria diagnostic laboratory of Jiangsu province carried out microscopic examination and PCR test in blood slides and blood samples. Plasmodium antigen detection was based on colloidal gold immunochromatography. The kit was produced by Guangzhou Wanfu Biotechnology Co. Ltd. A Roche Cobas E601 automatic electrochemical luminescence analyzer and an auxiliary reagent (Elecsys BRAHMS procalcitonin, Roche Diagnostics GmbH) were used to determinate the procalcitonin content of the serum. It was considered positive if the procalcitonin concentration was > 0.5 ng/mL.
3.2.1. Antimalarial Treatment Scheme
All patients received 80 mg (double for the first time) of artemether once a day through intravenous injection or intravenous drip, and subsequently received the quinine compound preparation. Then, symptomatic supportive treatment was performed, according to their respective complications.
3.2.2. Marker Observation
The procalcitonin and CRP markers were determined at 7 - 14 days’ hospitalization and 3 - 6 months’ follow-up after discharge, as follows: the content before antimalarial treatment, the minimum content within two weeks after treatment, and the content during recrudescence.
3.3. Statistical Processing
SPSS 19.0 software was used for the statistical analysis of data. The data with normal distribution were expressed as
4. Results
The malaria patients were all hospitalized. The procalcitonin level was significantly elevated in 95% (21/22) of the patients before antimalarial treatment while the procalcitonin level of one severe malaria patient reached up to 100 ng/mL. The procalcitonin level of all patients sharply declined with the improvement and cure of the disease after effective antimalarial treatment so that the procalcitonin levels were < 0.5 ng/mL and 0.5 - 0.8 ng/mL in 59% (13/22) and 23% (5/22) of the patients, respectively. The comparison of two inflammation markers before and after treatment showed statistically significant differences (P < 0.05, Table 1).
Comparison of Procalcitonin and CRP Markers of Malaria Patients Before and After Treatment (N = 22)
| Groups | Procalcitonin, ng/mL, M (P25, P75) | CRP, mg/L, |
|---|---|---|
| Before treatment | 3.28 (0.95, 13.11) | 99.63 ± 51.63 |
| After treatment | 0.48 (0.23, 0.84) | 20.08 ± 13.45 |
| Z/t value | 4.074 | 8.167 |
| P value | < 0.05 | < 0.05 |
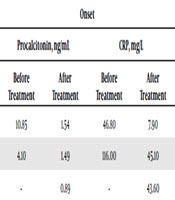
The follow-up period of patients lasted 3 - 6 months. Three patients suffered from the recrudescence. After the initial antimalarial treatment, the procalcitonin and CRP levels declined in patient 1 and patient 2. However, the procalcitonin level (> 1.0 ng/mL) failed to recover to the normal level within two weeks after treatment. The CRP level slightly increased. Patient 3 suffered from cerebral malaria. The patient recovered after the antimalarial treatment in the Chinese hospital in Africa (procalcitonin and CRP data were not available), but suffered from fever after returning home from abroad. The procalcitonin level was 0.89 ng/mL. Plasmodium was not found in the thick and thin blood smears through several microscopic examinations. The procalcitonin and CRP levels gradually increased through dynamic observation and the procalcitonin level reached 19.0 ng/mL when Plasmodium was found in the blood smear after two months (Table 2).
Distribution of Procalcitonin and CRP at Time Nodes of Recrudescence Patients
| Items | Onset | Recover | ||||||
|---|---|---|---|---|---|---|---|---|
| Procalcitonin, ng/mL | CRP, mg/L | Procalcitonin, ng/mL | CRP, mg/L | |||||
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | |
| Case 1 | 10.85 | 1.54 | 46.80 | 7.90 | 2.21 | 0.39 | 74.60 | 21.00 |
| Case 2 | 4.10 | 1.49 | 116.00 | 45.10 | 6.00 | 0.25 | 190.0 | 12.80 |
| Case 3 | - | 0.89 | - | 43.60 | 19.21 | 0.17 | 96.10 | 2.50 |
5. Discussion
As a propeptide substance of calcitonin, procalcitonin is an active ingredient secreted by thyroid C cells and formed by the hydrolysis of the proteolytic enzyme in cells. The procalcitonin level is very low in the serum of healthy people, and is generally not more than 0.30 ng/mL. However, it sharply increases (10, 11) in the case of whole or repeated local inflammation in the human body, especially following bacterial infections. Research has indicated (12-14) that the procalcitonin level in the serum can be used for the better assessment of patient’s condition and prognosis through dynamical observation. A high or rapidly increasing procalcitonin level often indicates a serious condition and poor prognosis, while a procalcitonin level that is low or declines rapidly often indicates a mild condition and good prognosis.
According to domestic and foreign scholars in recent years (15-17), the procalcitonin level also significantly increases in the pathogenetic process. The slow decline of high procalcitonin levels after treatment indicates a serious condition and poor prognosis, while a procalcitonin level that declines sharply after treatment can be taken as a marker for judging the curative effect. The present study also supports this idea. The procalcitonin level was significantly elevated in 95% (21/22) of patients before treatment, where the procalcitonin level of one patient reached up to 100 ng/mL. Patients who simultaneously suffer from hemolytic uremic syndrome in the course of disease and receive hemodialysis treatment for many times are severe malaria patients.
The malaria situation in China mainly manifests imported malaria, and the majority is falciparum malaria, which is due to infection from malaria endemic areas. Plasmodium falciparum can cause immune vascular injury and release cytokines such as TNF-α and IL-1, which directly stimulate and induce the release of procalcitonin. Severe cases may be related to the type, amount, and duration of exposure to Plasmodium parasites (18), leading to relatively severe damage and higher procalcitonin levels. A study by Hua-Yun et al. (19) on the malaria situation in Jiangsu showed that among 308 patients, only one patient was infected through blood transfusion, while the rest suffered from imported malaria, with falciparum malaria accounting for 77% (237/308) of the cases. Patients with falciparum malaria can more easily suffer from recrudescence, and a part of initial patients would suffer from malaria due to the mass propagation of a small amount of Plasmodium in the erythrocytic stage remaining in the body under certain conditions (20).
A study by Xie et al. (21) on the clinical features of 56 patients with imported malaria showed that 16.7% of the patients suffered from recrudescence after cure. The recrudescence mechanism of malaria is more complicated, and is generally considered to correlate with many factors, such as the species of infectious Plasmodium, antigenic variation of Plasmodium, non-standard use of antimalarial drugs, drug sensitivity, and decrease in the host’s specificity immunity (22). In recent years, many studies (23-25) have found that some malaria patients suffer from recrudescence due to the decreased sensitivity to artemisinin and resistance to drugs. During the recrudescence, missed diagnosis may be due to the low early positive rate of peripheral blood smear and different malaria cycles, time of blood sampling and inspection staff level. Plasmodium circulating antigen detection and PCR are difficult to be widely applied due to their technical difficulties.
In the practical clinical work, there is an urgent need for a more objective, convenient, and low-cost auxiliary diagnostic marker for the early determination of malaria recrudescence, providing an alert for clinicians, shortening the patient’s confirmation time, and allowing the patient to receive timely antimalarial treatment. There are many studies on procalcitonin respecting bacterial infections. Recent guidelines recommend procalcitonin levels of 0.5 ng/mL as a reference point for systemic bacterial infection (11). In terms of procalcitonin guidance for the use of antibiotics, studies (26, 27) recommended that the primary calcitonin concentration should be reduced by 80% or more than its peak value or reduced to 0.5 ng/mL or lower; then, antibiotics should stop. However, if the procalcitonin concentration is still > 0.5 ng/mL, maintenance or sequential treatment may be required. Whether there is the same reference significance for malaria patients, no relevant research report has been found.
It was found from our study that patients with a procalcitonin level of < 0.5 ng/mL and 0.5 - 0.8 ng/mL within two weeks after antimalarial treatment accounted for 59% and 23% of the patients, respectively, and they did not suffer from recrudescence during the follow-up visits. However, the minimum procalcitonin level in three patients who suffered from malaria recrudescence failed to recover to the normal level within two weeks after treatment and it was still > 0.8 ng/mL. In the present study, the investigators suggest that when procalcitonin levels remain > 0.8 ng/mL or increase gradually through dynamic observation within two weeks after antimalarial treatment, heightened alertness should be given to recrudescence, and blood smear microscopic examination should be carried out repeatedly to determine Plasmodium. It would also be necessary to adopt the nucleic acid probe and other techniques early.
5.1. Conclusions
Considering that the overall sample of the research was limited, when the procalcitonin level would be taken as the evaluation index for the prognosis of malaria recrudescence after antimalarial treatment, it would be necessary to enlarge the sample for further verification, and define a more reasonable threshold.
References
-
1.
WHO. World malaria report 2015. Geneva: World Health Orgnization; 2015. 157 p.
-
2.
Lubell Y, Blacksell SD, Dunachie S, Tanganuchitcharnchai A, Althaus T, Watthanaworawit W, et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect Dis. 2015;15:511. [PubMed ID: 26558692]. [PubMed Central ID: PMC4642613]. https://doi.org/10.1186/s12879-015-1272-6.
-
3.
Bruneel F, Tubach F, Mira JP, Houze S, Gibot S, Huisse MG, et al. Imported falciparum malaria in adults: host- and parasite-related factors associated with severity. The French prospective multicenter PALUREA cohort study. Intensive Care Med. 2016;42(10):1588-96. [PubMed ID: 27169586]. https://doi.org/10.1007/s00134-016-4356-x.
-
4.
Szalai AJ, Barnum SR, Ramos TN. Deletion of C-reactive protein ameliorates experimental cerebral malaria? Trans R Soc Trop Med Hyg. 2014;108(9):591-3. [PubMed ID: 25002461]. [PubMed Central ID: PMC4192040]. https://doi.org/10.1093/trstmh/tru098.
-
5.
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515-8. [PubMed ID: 8094770]. https://doi.org/10.1016/0140-6736(93)90277-n.
-
6.
Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J Infect Dis. 2000;181(1):176-80. [PubMed ID: 10608764]. https://doi.org/10.1086/315214.
-
7.
Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit Care Med. 2006;34(7):1996-2003. [PubMed ID: 16715031]. https://doi.org/10.1097/01.CCM.0000226413.54364.36.
-
8.
Hunziker S, Hugle T, Schuchardt K, Groeschl I, Schuetz P, Mueller B, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am. 2010;92(1):138-48. [PubMed ID: 20048106]. https://doi.org/10.2106/JBJS.H.01600.
-
9.
National Health and Family Planning Commission of the People's Republic of China. Industry standard WS259-2015 of the People's Republic of China. Diagnosis of Malaria. Notice No. 18 of the National Health and Family Planning Commission of the People’s Republic of China. 2016. China: National Health and Family Planning Commission of the People's Republic of China; 2016.
-
10.
Saikant R, Ravindran S, Vijayan A, Maya V, Lakshmi S, Kartik R, et al. Response of letter to the editor on procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:68. [PubMed ID: 29234495]. [PubMed Central ID: PMC5719722]. https://doi.org/10.1186/s40560-017-0260-x.
-
11.
Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-73. [PubMed ID: 24982830]. [PubMed Central ID: PMC4071182]. https://doi.org/10.3343/alm.2014.34.4.263.
-
12.
Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: A systematic review and meta-analysis. Int J Infect Dis. 2015;38:68-76. [PubMed ID: 26188130]. https://doi.org/10.1016/j.ijid.2015.07.011.
-
13.
Jimeno A, Garcia-Velasco A, del Val O, Gonzalez-Billalabeitia E, Hernando S, Hernandez R, et al. Assessment of procalcitonin as a diagnostic and prognostic marker in patients with solid tumors and febrile neutropenia. Cancer. 2004;100(11):2462-9. [PubMed ID: 15160353]. https://doi.org/10.1002/cncr.20275.
-
14.
Carannante N, Rossi M, Fraganza F, Coppola G, Chiesa D, Attanasio V, et al. A high PCT level correlates with disease severity in Plasmodium falciparum malaria in children. New Microbiol. 2017;40(1):72-4. [PubMed ID: 28217817].
-
15.
Righi E, Merelli M, Arzese A, Siega PD, Scarparo C, Bassetti M. Determination of PCT on admission is a useful tool for the assessment of disease severity in travelers with imported Plasmodium falciparum malaria. Acta Parasitol. 2016;61(2):412-8. [PubMed ID: 27078668]. https://doi.org/10.1515/ap-2016-0055.
-
16.
Diez-Padrisa N, Bassat Q, Roca A. Serum biomarkers for the diagnosis of malaria, bacterial and viral infections in children living in malaria-endemic areas. Drugs Today (Barc). 2011;47(1):63-75. [PubMed ID: 21373650]. https://doi.org/10.1358/dot.2011.47.1.1534821.
-
17.
Hesselink DA, Burgerhart JS, Bosmans-Timmerarends H, Petit P, van Genderen PJ. Procalcitonin as a biomarker for severe Plasmodium falciparum disease: A critical appraisal of a semi-quantitative point-of-care test in a cohort of travellers with imported malaria. Malar J. 2009;8:206. [PubMed ID: 19723338]. [PubMed Central ID: PMC3224901]. https://doi.org/10.1186/1475-2875-8-206.
-
18.
[No author listed]. Severe falciparum malaria. World Health Organization, communicable diseases cluster. Trans R Soc Trop Med Hyg. 2000;94 Suppl 1:S1-90. [PubMed ID: 11103309].
-
19.
Hua-Yun Z, Wei-Ming W, Guo-Ding Z, Yuan-Yuan C, Ya-Ping G, Sui X, et al. [Epidemiological analysis of malaria prevalence in Jiangsu province in 2016]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(1):32-6. Chinese. [PubMed ID: 29536704]. https://doi.org/10.16250/j.32.1374.2017236.
-
20.
Richter J, Franken G, Holtfreter MC, Walter S, Labisch A, Mehlhorn H, et al. Clinical implications of a gradual dormancy concept in malaria. Parasitol Res. 2018;117(7):2139-48. [PubMed ID: 29728826]. https://doi.org/10.1007/s00436-018-5901-z.
-
21.
Xie QX, Han H, Xu YY, Wei SF, Jiang XP, Li X. Clinical characteristics analysis of 56 imported malaria cases [J]. Chin J Dis Control Prev. 2011;6:538-40.
-
22.
Claser C, Chang ZW, Russell B, Renia L. Adaptive immunity is essential in preventing recrudescence of Plasmodium yoelii malaria parasites after artesunate treatment. Cell Microbiol. 2017;19(11). [PubMed ID: 28664674]. https://doi.org/10.1111/cmi.12763.
-
23.
Gobbi F, Buonfrate D, Menegon M, Lunardi G, Angheben A, Severini C, et al. Failure of dihydroartemisinin-piperaquine treatment of uncomplicated Plasmodium falciparum malaria in a traveller coming from Ethiopia. Malar J. 2016;15(1):525. [PubMed ID: 27809844]. [PubMed Central ID: PMC5094029]. https://doi.org/10.1186/s12936-016-1572-3.
-
24.
Singh RK. The response of a case of multidrug-resistant, Plasmodium falciparum malaria to an unusual combination of antimalarial drugs. Ann Trop Med Parasitol. 2002;96(4):417-8. [PubMed ID: 12171623]. https://doi.org/10.1179/000349802125001168.
-
25.
Witkowski B, Khim N, Kim S, Domergue A, Duru V, Menard D. [Multiple and successive treatment failures in a patient infected by Plasmodium falciparum in Cambodia and treated by dihydroartemisinin-piperaquine]. Bull Soc Pathol Exot. 2016;109(2):87-90. [PubMed ID: 27100863]. https://doi.org/10.1007/s13149-016-0487-4.
-
26.
Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: A randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93. [PubMed ID: 16603606]. https://doi.org/10.1164/rccm.200512-1922OC.
-
27.
de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27. [PubMed ID: 26947523]. https://doi.org/10.1016/S1473-3099(16)00053-0.