Abstract
Context:
Five species of the genus Ajuga (Lamiaceae) having the common name of "bugle" are found in Iran. In Persian medicine (PM), the genus Ajuga (Kamaphytus) is used for treating jaundice, joint pain, gout, amenorrhea, sciatica, and wound healing. This study aimed to review the ethnobotanical, phytochemical, and biological activities of Ajuga species that grow in Iran to determine their therapeutic potentials and suggest further studies on the healing properties of this genus in Iran.Evidence Acquisition:
Electronic databases such as PubMed, Scopus, and Google Scholar were comprehensively searched for studies on Ajuga species in Iran, including "Ajuga austro-iranica," "Ajuga chamaecistus," "Ajuga comata" (Syn.: "Ajuga chia," "Ajuga chamaepitys subsp. Chia"), "Ajuga orientalis," and "Ajuga reptans." The search period was from 1966 to February 2021. The related articles were selected according to the inclusion and exclusion criteria of the current study.Results:
Several ethnobotanical and pharmacologic reports have verified the traditional uses of the genus Ajuga for anti-inflammatory, hypoglycemic, hypolipidemic, analgesic, anabolic, anti-arthritis, antipyretic, and hepatoprotective activities. Numerous phytochemicals have been identified from Ajuga species involving phytoecdysteroids, neo-clerodane-diterpenes, iridoids, flavonoids, withanolides, phenylethyl glycoside, and essential oils.Conclusions:
Due to the beneficial therapeutic effects of Ajuga genus, it can be considered in future clinical studies as a source of natural antioxidants, dietary supplements in the pharmaceutical industry, and stabilizing food against oxidative deterioration.Keywords
Ajuga Persian Medicine Pharmacological Activities Phytochemical Compositions Ethnobotanical Uses
1. Context
The genus Ajuga of the Lamiaceae family, commonly known as bugle or bugleweed, ground pine, and carpet bugle, is a large plant genus that grows in Europe, Asia, Africa, Australia, and North America. The genus Ajuga is one of the 266 genera of the family Lamiaceae. Five species of the genus Ajuga are growing in the plant flora of Iran, including Ajuga austro-iranica Rech f., Ajuga chamaecistus Ging., Ajuga comata Stapf. (Syn.: Ajuga chia, Ajuga chamaepitys subsp. chia), Ajuga orientalis L., and Ajuga reptans L. (1-8). Many plants of the Ajuga genus are used traditionally as a remedy for rheumatic fever, dysentery, malaria, hypertension, diabetes, and gastrointestinal ailments, in addition to anti-inflammatory, astringent, diuretic, and antifungal activities (1, 2, 8-11). Different phytochemical compounds have been isolated and identified from several species of this genus, including phytoecdysteroids (12, 13), diterpenoids (14), and iridoids (8, 12, 15). Numerous biological studies showed that some of these compounds have beneficial biological effects such as antibacterial, antifungal, anti-plasmodial, cytotoxic, antitumor, analgesic, anti-inflammatory, antidiabetic, and antioxidant effects (16). In this review, Ajuga plants growing in Iran have been studied for their ethnobotanical, phytochemical, and pharmacological properties.
2. Evidence Acquisition
Electronic databases including PubMed, Scopus, and Google Scholar were explored for investigating Ajuga. The search period was from 1966 to February 2021. The search keywords included "Ajuga," "Ajuga austro-iranica," "Ajuga chamaecistus," " Ajuga comata," "Ajuga chia," "Ajuga pseudochia," "Ajuga chamaepitys subsp. chia", "Ajuga orientalis," and "Ajuga reptans." Inclusion criteria were in vitro, in vivo, or phytochemical evaluation and traditional and ethnobotany use of five Iranian Ajuga species in papers with available full texts in English. Exclusion criteria were review articles and papers with non-English full-texts.
3. Results
3.1. Traditional and Ethnobotanical Uses
The genus Ajuga (Kamaphytus) is used traditionally for the management of jaundice, joint pain, gout (2, 17, 18), amenorrhea, sciatica, and wound-healing in traditional Persian medicine (19, 20). It is also utilized as a carminative and diuretic agent (3, 10, 20, 21). Iranian physicians believe that this plant can be effective for liver (22, 23) and spleen disease. They also used this remedy for treating constipation (24). The critical point of "Kamaphytus" mentioned in many books of Persian physicians is the wound-healing effects of these plants (20, 21, 24-27).
Table 1 shows the traditional uses of some species of the genus Ajuga growing in different regions of the Iranian plateau. Most species of this genus are used for treating gastrointestinal diseases such as constipation, skin diseases, blood sugar control, rheumatism, menstrual cramps, hypertension, gout, and wound healing (28).
Traditional Uses, Pharmacological, and Clinical Studies of Ajuga Genus Grown in Iran
| Plant Name | Traditional Use | Region | Pharmacological and Clinical Studies | References |
|---|---|---|---|---|
| Ajuga orientalis L. | Skin diseases, diabetes, digestive, rheumatism | Anatolia, Palestinian area | Antibacterial and antioxidant effect, plant antifungal | (6, 29, 30) |
| Ajuga austro-iranica Rech.f. | Women infertility, gynecological problems, cardiac problems, hypertension, constipation | Kohghiluyehva Boyer Ahmad | - | (30) |
| Ajuga comate synonym of Ajuga chamaepitys subsp. Chia (Schreb.) Arcang. | Cancer antiarthritic external, wound healing, diarrhea, hemorrhoids, and various intestinal infections | Iran, Eurasia, Jordan, Europe, Turkey | Antibacterial effect | (22, 31) |
| Ajuga chamaepitys (L.) Schreb. subsp. Chia (Schreb.) Arcang. Var. ciliate Briq. | Menstruation, diaphoretic | Turkey | Anticolitis effect | (32, 33) |
| Ajuga chamaepitys | Diuretic, tonic, emmenagogue agent for wound-healing and perspiration; treating scorpion and snake bites, hemorrhoids, stomachache, jaundice, inflammatory diseases, such as gout and joint pains, and common colds; antimicrobial, antiviral, and antifeedant | Iran | Antioxidant activity, antiproliferative activity, analgesic effect | (17, 34-38) |
| Ajuga chamaecistus | Treatment of edema, jaundice, joint pains, and sciatica. Topically, it has been used for wound healing, and breast hardness; diuretic and emmenagogue agent | Iran | Ajuga chamaecistus ssp. tomentella: Anti-inflammatory and analgesic, cytotoxic, antibacterial, antioxidant, blood sugar lowering, toxicity studies larvicidal, antibacterial; Ajuga chamaecistus subsp. scoparia: Antimicrobial effect antidiabetic, skincare, and neuroprotective effect | (2, 3, 8-11, 23, 39, 40) |
| Ajuga reptansL. | Anti-inflammatory, wound healing, hepatoprotective properties, mild analgesic | The center and, especially, the eastern part of Europe, northern Iran, Caucasus | Antioxidant and antibacterial effect, antifeedant effect; anti-colitis effect | (41-44) |
3.2. Phytochemicals
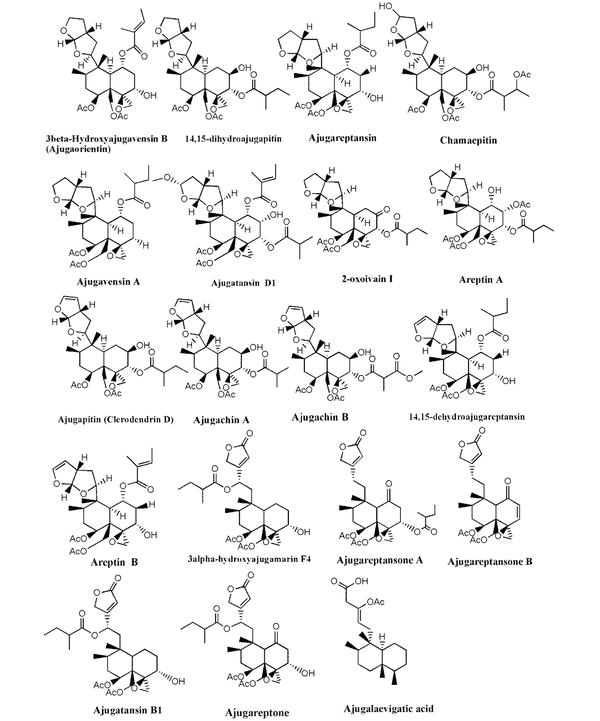
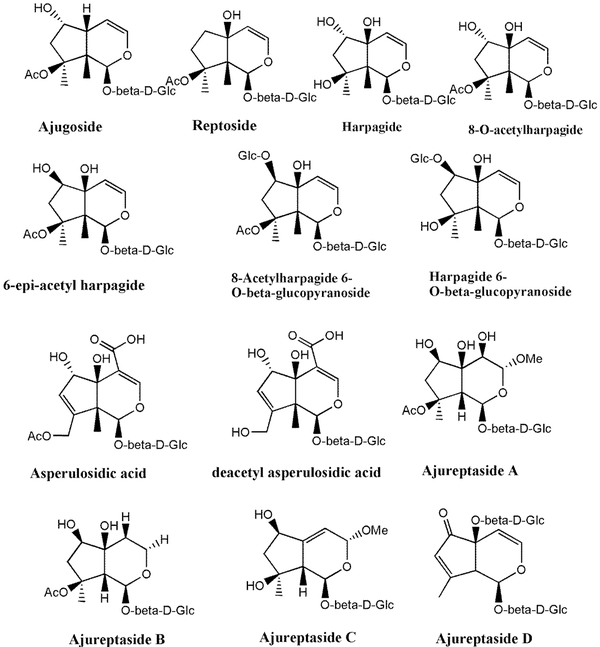
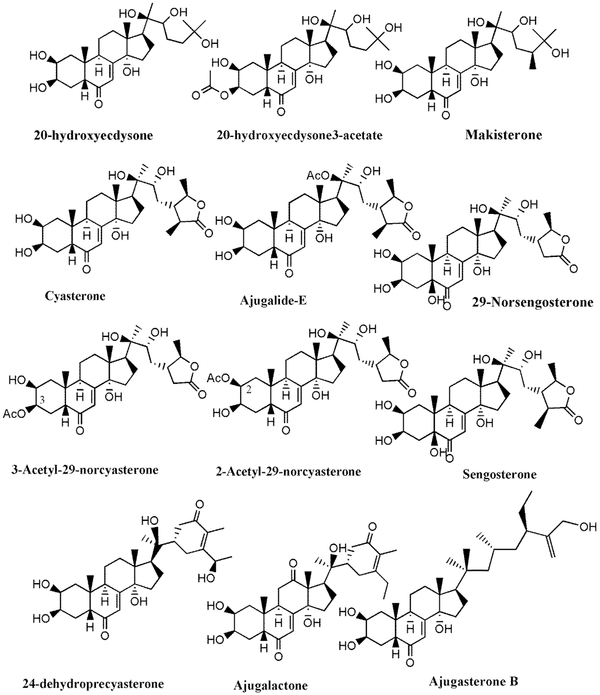
Much phytochemical research has assessed the separation of Ajuga ingredients. These studies have led to the isolation of several secondary metabolites, including neo-clerodane_diterpenes and diterpenoids, phytoecdysteroid components, flavonoid derivatives, iridoids, sesquiterpenoid compounds, withanolides, phenylethanoid glycoside derivatives, sterols, anthocyanins, and O-coumaric acid compounds (3, 8, 12, 13, 15, 23, 29, 34, 39, 40, 45-51). Isolated compounds from different Ajuga species growing in Iran are shown in Table 2. Also, the phytochemical structures of the main secondary metabolites of Ajuga species in Iran are presented in Figures 1 - 3. According to the results, phytochemical compounds have been determined in several species, especially A. chamaecistus, A. chamaepitys, and A. reptans. The major constituents in different species were phenolic compounds like phenylethanoid glycosides, neo-clerodane-type diterpenes, iridoids, anthocyanin glycosides, and phytoecdysteroids. Phytochemical studies of A. austro-iranica and some subspecies have not been reported and need to be studied.
Phytochemicals from Ajuga Species Growing in Iran
| Ajuga Species | Chemical Compound | Part Used | References |
|---|---|---|---|
| Ajuga orientalis L. | Neo-clerodane-type diterpenes: Ajugorientin | Aerial parts | (45) |
| Ajuga chamaepitys | Phenylethanoidglycoside: Acteoside; flavone glycosides: Chrysoeriol 7-Oglucopyranoside; (3'-methoxy-luteolin 7-O-glucopyranoside) and apigenin 7-Orhamnopyranoside, isovitexin, orientin, flavonol, and cyaniding; iridoid: Ajugoside, reptoside, 8-O-acetylharpagide, harpagide, 6-O-β-D-glucopyranosyl-harpagide, asperulosidic acid and deacetylasperulosidic acid. 6-O-β-, D-glucopyranosyl-8-O-acetylharpagide; phytoecdysteroids: Cyasterone, ecdysterone (20-hydroxyecdysone); neo-clerodanediterpenoids: ajugapitin (Clerodendrin D), 14,15-dihydroAjugapitin, chamaepitin, ajugachin A, ajugachin B, ajugalaevigatic acid | Aerial parts; flowering aerial; parts and root | (35, 36, 51, 52) |
| Ajuga chamaepitys subsp. chia ( syn. A. comata) | Neo-clerodanediterpenoids; ajugachin A, ajugachin B, ajugapitin and 14,15_dihydroajugapitin; iridoidglycoside: Ajugoside, asperulosidicacid and deacetyl-asperulosidic acid | Aerial parts | (32, 53) |
| Ajuga chamaecistus ssp. tomentella | Coumaric acid derivative: Cis-melilotoside and trans-melilotoside; phenylethanoid glycosides: Lavandulifolioside, leonoside B, martynoside; ecdysteroids: 20-hydroxyecdysone, cyasterone, ajugalactone, makisterone A, 24-dehydroprecyasterone, ajugalide-E; iridoids: 8-acetylharpagide | (11, 23) | |
| Ajuga reptans L. | Anthocyanins: Delphinidin 3-(p-coumaroylferuloyl) sophoroside-5-malonylglucoside, delphinidin 3-(diferuloyl) sophoroside-5-malonylglucoside, cyanidin 3-(di-p-coumaroyl) sophoroside-5-glucoside, delphinidin 3-(diferuloyl) sophoroside-5-glucoside and cyanidin 3-(feruloyl-p-coumaroyl) sophoroside-5 malonylglucoside; iridoidglucosides: Ajureptaside A, Ajureptaside B, ajureptaside C, ajureptaside D, reptoside, harpagide, 6-epi-acetyl harpagide, acetyl harpagide; aliphatic alcohol glycoside: 1-octen-3-ol 3-O-glucopyranosyl-(1→2)-(48)-glucopyranoside; ecdysteroids: ajugalactone, 20-hydroxyecdysone, 20-hydroxyecdysone; 3-acetate, 29-Norsengosterone,2-Acetyl-29-norcyasterone, 3-Acetyl-29-norcyasterone,Sengosterone, ajugasterone B; neo-clerodanediterpenoids:14,15-dehydroajugareptansin, 3β-hydroxy ajugavensin B and 3α-hydroxy ajugamarin F4,ajugareptansin, ajugareptansone A, B, ajugavensin A, ajugatansin B1, D1, ajugareptone, ajugalaevigatic acid, 2-oxoivain I, areptin A, B,ajugaorientin,ajugachin A; abietanes: ajugaside A; phenylpropanoidglycosides: Teupolioside | (48, 51, 54, 55) |
Chemical structure of neo-clerodane diterpenoids identified in Ajuga species growing in Iran
Chemical structure of iridoids identified in Ajuga species growing in Iran
Chemical structure of phytoecdysteroids identified in Ajuga species growing in Iran
Moreover, the constituents of volatile oil isolated from the aerial parts of the Ajuga plant (leaves and flowers) are shown in Table 3. Phytochemical studies of essential oils of almost all species of Iran have been performed, and the major compounds in different species were identified, such as germacrene-D, α- and β-pinene, 1-octen-3-ol, p-cymene, thymol, and hexadecanoic acid.
| Ajuga Species | Part Used | Method of Extraction-Method of Identification | Main Components (%) (K.I.) | References |
|---|---|---|---|---|
| Ajuga orientalis L. | Aerial parts | Hydrodistillation by Clevenger-type apparatus for 3 h-GC/MS | Germacrene-D (24.2%) (1480), β-cubebene (18.3%) (1393), β-caryophyllene (16.9%) (1418),α-cubebene (5.3%) (1349), β-selinene (4.5%) (1489), bicyclogermacrene (4.4%) (1496) andα-humulene (4.2%) (1452) | (56) |
| Ajuga austro-iranica | Aerial parts | Hydrodistillation by Clevenger-type apparatus for 4 h-GC/MS | Trans-Verbenol (a pinene-type Monoterpenoid)(7%) (1144), caryophyllene oxide (6.8%) (1581), 6,10,14,-trimethyl-2-pentadecanone(6.5) (1844), myrtenol(6.3) (1196), 1-octen-3-ol (6.2) (980), β-pinene (6.1) (979),verbenone (5.7) (1208) | (57) |
| Ajuga chamaepitys | Aerial parts | Hydro-distillation method by the Clevenger apparatus for about 4 h,- GC-MS and GC-FID | α-pinene (23.66%) (931), β-pinene (9.33%) (971), 1-octen-3-ol (9.72%) (971), β-phellandrene (8.70%) (1022) and germacrene-D (7.92%) (1477) | (36) |
| Ajuga chamaepitys | Fresh aerial parts | Hydrodistillation-GC/FID and GC/MS | The monoterpene hydrocarbons α-pinene (16.1%) (936) and β-pinene (34.38) (981), γ-terpinene (7.7%) (1062), limonene (6.1%) (1032) and mycrene (1.4%) (994) The main sesquiterpene hydrocarbons identified were germacrene D (5.6%) (1487), γ-elemene (3.7%) (1439), β-cubebene (1.7%) (1396), α-copaene (1.8%) (1382) and β-bourbonene (1.5%) (1392) | (37) |
| Ajuga chamaepitys | Fresh flowering aerial parts | Aerial parts, hydrodistillation by a Clevenger type apparatus -GC-FID and GC-MS | Germacrene D (13.4%), kaurene (8.3%) and (E)-phytol (5.3%), ethyl linoleate (13.7%), and β-pinene (6.8%), oxygenated monoterpenes (4.1%), linalool (3.2%), globulol (3.3%), and 1-octen-3-ol (4.9%) | (36) |
| Ajuga chamaecistus subsp. scoparia | Crushed flowers | SDE (simultaneous distillation–extraction) and Clevenger apparatus for 3.5 h. | β-pinene (23.5%) (993), α-pinene (6.9%) (944), limonene (10.8%) (1042), linalool (8.3) (1113) and eugenol (7.7%) (1373). | (9) |
| Ajuga chamaecistus subsp. scoparia | The aerial parts | Hydrodistillation using a Clevenger-type apparatus for 3.5 h. | β-pinene (16.08%) (981), α-thojene (10.66%) (926), α-pinene (7.43%) (939), linalool (7.37%) (1101), bicyclogermacrene (6.71%) (1494), δ-cadinene (6.40%) (1538), limonene (4.95%) (1033) and spathulenol (3.09%) (1581) | (4) |
| Ajuga chamaecistus Ging. subsp. tomentellaRech. f. | The aerial parts | Hydrodistillation using a Clevenger-type apparatus for 4 h-GC/MS | Thymol (34.45 %) (1288), exo-fenchol (15.58 %) (1120), β-pinene (8.26 %) (974), 1-octen-3-ol (5.92 %) (975), α-terpineol (3.88 %) (1158), 2-hexanol (3.85 %) (850), α-thujene (2.66%) (928), and α-pinene (2.54 %) (935). | (40) |
| Ajuga reptans L. | The dried flowers and leaves | Hydrodistillation | Flowers: Hexadecanoic acid (38.0%) (1969), 6,10,14-trimethylpentadecan-2-one (16.4%) (1846), n-tetradecane (13.0%) (1400), and (Z,Z,Z)-9,12,15-octadecatrienoic acid methyl ester (7.3%) (2135). Leaves: 1-octen-3-ol (55.6%) (979), hexadecanoic acid (10.7%) (1969), terpinolene (5.6%) (1089), and 6,10,14-trimethyl-2-pentadecanone (5.2%) (1846). | (58) |
| Ajuga reptans L. | Fresh aerial parts | Hydrodistillation using a Clevenger type apparatus for 4 h-GC/MS | 1-octen-3-ol (40.7 %) (979), linalool (13.7%) (1101), n-hexadecanoic acid (11.7%) (1973), n-heptacosane (5.2%) (2700), 2-methylbenzofuran (4.6%) (1166) | (59) |
3.2.1. Biological Importance of Phytochemicals Identified in Ajuga Species
3.2.1.1. Neo-clerodane Diterpenes
Neo-clerodane diterpenes are the characteristic type of phytochemicals from the genus Ajuga L. besides valuable pharmacological properties (28). Neo-clerodane diterpens identified in Ajuga species from Iran are represented in Table 2 and Figure 1. Some biological activities of neo-clerodane diterpens have been reported like moderate neuroprotective (60), antifeedant (41), antiproliferative (61), anti-inflammatory (62, 63), antinociceptive (64), and vasorelaxant effects (65). Also, inhibitory activities on LPS-induced NO production (66) and RANKL-induced Osteoclastogenesis (67) have been shown in previous studies.
3.2.1.2. Iridoids
Iridoids are a group of secondary metabolites with a wide range of biological activities present in the genus Ajuga L. (68). Among these compounds, 8-acetylharpagide has been reported to show a strong antitumor-promoting activity (69). Also, iridoids such as ajugoside, asperulosidic acid, and deacetyl-asperulosidic acid isolated from A. chamaepitys subsp. chia showed antiprotozoal activity (70). In addition, 6-deoxyharpagide exhibited anti-inflammatory effects via COX-2 inhibition (71). Chemical structures of isolated iridoids from Ajuga species in Iran are illustrated in Figure 2 and Table 2.
3.2.1.3. Phytoecdysteroids
The ecdysteroids are a class of polyhydroxysteroids existing in the genus Ajuga. This group of secondary compounds exhibits a wide range of biological activities in mammals and adaptogenic, anabolic (72), hypoglycemic (73), hepatoprotective, immunoprotective, wound-healing (72), and free radical scavenging effects (74). Besides, 20-Hydroxyecdysone, cyasterone, and ajugalactone are the most common phytoecdysteroids in Ajuga specie (75).
3.3. Biological Effects
3.3.1. Ajuga chamaecistus
Ajuga chamaecistus is the most extensive species of Ajuga in Iran, with four subspecies including scoparia, euphrasioides, chamaecistus, and tomentella that are all exclusive to Iran. The plant is distributed in Afghanistan, Central Asia, eastern Turkey, the Caucasus, and Iraq and usually grows on mountainous or rocky slopes. Also, A. chamaecistus subsp. scoparia is a habitat of Iran's western, central, and southern provinces. Moreover, A. chamaecistus subsp. euphrasioides grows in central Iran and A. chamaecistus subsp. chamaecistus grows in the northwestern, western, and central provinces of Iran.
In addition, A. chamaecistus subsp. tomentella, also known as Sefid Moshkak and Mash Daro, is a shrub and perennial herb that grows mainly in mountainous areas and sporadically in other steppe and semi-steppe areas. Its habitat is located in the west, center, and south of Iran (2, 4, 76).
3.3.1.1. Ajuga chamaecistus Ging. ssp. tomentella
3.3.1.1.1. Anti-inflammatory and Analgesic Effects
As demonstrated in the study by Khanavi et al., the oral administration of different doses of methanolic and aqueous extracts and their fractions (200, 400, and 600 mg/kg) had analgesic effects in the chronic phase (15 - 60 min after formalin injection) in mice. Besides, total water and diethyl ether extracts at a dose of 400 mg/kg presented a very significant analgesic action (2).
3.3.1.1.2. Cytotoxic Effects
In the study by Sadati et al., the cytotoxic effect of the main compounds isolated from plant methanolic extract, including 20-hydroxyecdysone, cyasteron, and 8-acetylharpagide, on cancer cells (T47D, Caco-2 , and HT-29) and normal cells (NIH 3T3) was investigated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay. It was found that the main components of this plant had no cytotoxic effects up to 400 μg/mL (23).
3.3.1.1.3. Antibacterial Effects
In another study, the antibacterial effect of different extracts and fractions of A. chamaecistus ssp. tomentella was investigated on Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aerorginas. All fractions were effective on the tested bacterial species. The diethyl ether fraction had the greatest effect on Staphylococcus and Bacillus species. The greatest effect of butanol fraction was on E. coli (8).
In the study by Shams Ardekani et al., the antibacterial effect of volatile oil from aerial parts of A. chamaecistus subsp. chamaecistus was assessed using the disk diffusion process against Gram-positive bacteria (S. aureus and B. subtilis) and Gram-negative bacteria (E. coli and P. aeruginosa). Results displayed no considerable antibacterial activities against Gram-positive and Gram-negative bacteria, with 25 microliters per liter (40).
3.3.1.1.4. Antioxidant Effects
In the study of Sadati Lamardi et al., the antioxidant effect, free radical scavenging activity, and total phenolic content of aqueous and methanolic extracts of aerial parts of the plant were investigated using ferric reducing antioxidant power, DPPH (diphenyl-picryl-hydrazyl) test, and Folin-Ciocalteu methods. The results showed that the butanol fraction had the highest phenolic content (26.5 mg GAE/g of extract) and the highest antioxidant power (346.7 mmol FeІІ/g of extract) and inhibited DPPH free radicals (IC50 = 15.34 μg/mL) (10).
3.3.1.1.5. Blood Sugar Lowering Effect
In another study, the hypoglycemic effects of aqueous and methanol extracts of this plant were investigated in the model of induced diabetes using streptozotocin in Syrian mice. The results showed that all doses of the extract (200, 400, and 800 mg/kg) in oral administration reduced plasma glucose on days 3, 14, and 28 of the study. A dose of 400 mg/mL of the methanolic extract more greatly decreased glucose levels on the 14th day than metformin (500 mg/kg) (10).
3.3.1.1.6. Toxicity Studies
Acute and chronic toxicity of hydroalcoholic extract of this plant were investigated in rat. The acute toxicity study showed that the ethanolic extract was non-toxic up to a dose of 6,000 mg/kg. Based on the results of sub-chronic toxicity, after using the extract (1000 mg/kg) for 23 and 45 days, a significant reduction was observed in cholesterol and triglycerides. Histopathology of animal tissues did not show significant differences in animal tissues after 23 and 45 days between the treated and control groups (10).
3.3.1.1.7. Larvicidal Effect
In a study by Khanavi et al., the lethal effect of different fractions of methanolic extract of the plant was investigated on the malaria vector Anopheles stephensi larvae. The results showed that the hexane fraction of the extract could kill 100% of the larvae at a concentration of 102 ppm (11).
3.3.1.2. Ajuga chamaecistus subsp. scoparia
3.3.1.2.1. Antimicrobial Effect
Haghir Ebrahimabadi et al. reported the antimicrobial effect of essential oil prepared from flowering samples of A. chamaecistus subsp. scoparia collected from the Kashan area by agar disk diffusion method against 12 microorganisms, including Gram-negative and Gram-positive bacteria P. aeruginosa, E. coli, B. subtilis, S. aureus, K. pneumonia, S. epidermidis, S. dysenteriae, P. vulgaris, and Salmonella paratyphi-A serotype. Plant essential oil showed significant antimicrobial activity against all microorganisms. The minimum inhibitory concentration (MIC) values for microbial strains were 125 - 4000 μg/mL. The essential oil of this plant containing β-pinene, α-pinene, limonene, linalool, and eugenol as the main components showed significant antimicrobial potency compared to positive control antibiotics (9). Mohammadi-Bazargani et al., in an in vitro study, examined the antibacterial activity of the essential oil of this plant. The results exhibited antibacterial action on both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria, while hexane extract showed antibacterial effects only against S. aureus (39).
3.3.1.2.2. Antidiabetic, Skin Care, and Neuroprotective Effect
Movahhedin et al. evaluated antidiabetic, cholinesterase inhibitory, tyrosinase inhibitory, and antioxidant effects of essential oils and different extracts of A. chamaecistus subsp. scoparia, and identified their chemical compositions. The highest inhibitory effect on sugar-degrading enzymes was observed by volatile oils. The results exhibited that the essential oil significantly reduced the activity of α-glucosidase (equivalent to 4.3 mmol of acarbose per gram), α-amylase (2.8 mmol equivalents of acarbose per gram), acetyl cholinesterase (1.96 mg equivalent of gallic acid per gram), butyrylcholinesterase (2.2 mg gallic acid equivalent per gram), and tyrosinase (36 mg equivalent of kojic acid per gram); however, ethanolic and aqueous extracts displayed moderate effects on enzyme activity. Twenty-three phenolic compounds were extracted from the plant, and p-coumaric acid was the major component in both extracts. In addition, gallic acid and ferulic acid were the main compounds in aqueous and ethanolic extracts, respectively. Volatile oils of this plant showed longer anticholinesterase activity than aqueous decoction and ethanolic extract of the plant, so it may be used to treat Alzheimer’s disease since the plant has high radical scavenging and metal chelating activity. Therefore, it has a good antioxidant capacity and can be used in skin problems (3).
3.3.2. Ajuga chamaepitys (L.) Schreb.
3.3.2.1. Antiproliferative Effect
The cytotoxicity effects of A. chamaepitys essential oil, ethanolic, and aqueous extracts were tested by the MTT assay against human malignant melanoma (A375), human breast adenocarcinoma (MDA-MB 231), and human colon carcinoma (HCT116) cell lines. The results of this study showed that essential oil exhibited a moderate cytotoxic effect on MDA-MB 231, HCT116, and A375 cells with IC50s of 59.24, 64.12, and 67.44 μg/mL, respectively, while ethanolic extract was more effective on MDA-MB-231 than HCT116 cell line with IC50s of 36.88 and 60.48 μg/mL, respectively (35).
3.3.2.2. Analgesic Effect
In a study, Jaffal et al. investigated the analgesic effect of A. chamaepitys methanolic extract growing in Jordan with chemical and thermal pain-induced models in mice. The results showed that the extract of this plant significantly reduced the number of writhes in mice, comparable with the negative control group. The inhibitory effect of 300 mg/kg i.p. of the extract was similar to that of 300 mg/kg aspirin. Administration of 450 mg/kg (i.p.) of plant extract significantly reduced paw licking time in the early and late stages of the formalin test. The delay time in the hot plate test was also increased. Before the treatment, Naloxone injection having the extract reduced the analgesic effect of the extract. As a result, the analgesic effect of this plant can be affected by its effect on opioid receptors. The LC-MS analysis of the extract led to the identification of 19 compounds, the most important of which were isovitexin, orientin, flavonol, and cyanidin (52).
3.3.2.3. Antioxidant Activity
Venditti et al. studied the free radical scavenging activity of the essential oil, aqueous, and ethanolic extracts of A. chamaepitys aerial branches utilizing DPPH and ABTS+ free-radical-scavenging assays. According to the results, aqueous and ethanolic extracts displayed comparable radical scavenging properties in ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) test with IC50 = 50.0 and 54.9 μg/mL, about 27 folds higher than that of Trolox. Additionally, both aqueous and ethanolic extracts and the essential oil exhibited moderate antioxidant effects on DPPH radicals with IC50s from 83 to 300 times higher than that of Trolox (35). In the study by Delazar et al., the free-radical-scavenging potential of the extracts, fractions, and isolated compounds from A. chamaepitys was tested by the DPPH assay. The methanol extract presented the highest free-radical-scavenging activity (RC50: 1.15 × 10-1 mg/mL), and chrysoeriol 7-O-glucopyranoside was the most effective compound (RC50: 3.00 × 10-3 mg/mL) (36). Based on the antioxidant properties of A. chamaepitys, this plant can be suggested as a natural source of antioxidants, dietary supplements in pharmaceutical manufacturing, and stabilizing food against oxidative deterioration (34).
3.3.3. A. chamaepitys subsp. euphratica
3.3.3.1. Antimicrobial Activity
Turkoglu et al. examined the methanol, water, and chloroform extracts of A. chamaepitys subsp. euphratica using the disk diffusion method for antimicrobial properties. They tested the extracts on the growth of six bacterial species E. coli, S. aureus, B. cereus, P. aeruginosa, K. pneumoniae, and E. aerogenes, and two yeast species. The extracts showed antimicrobial effects on Gram-negative bacteria (inhibition zone diameter: E. coli 13 mm, P. aeruginosa 15 mm, K. pneumoniae 15 mm, and E. aerogenes 12 mm); however, they did not show any antimicrobial activity against Gram-positive bacteria and yeasts Saccharomyces cerevisiae and Candida sp. (34).
3.3.3.2. Antioxidant Activity
Several studies reported the antioxidant action of extracts and phenolic compounds of A. chamaepitys from different countries using the ABTS and DPPH antioxidant tests. Turkoglu et al. evaluated the antioxidant power of A. chamaepitys subsp. euphratica using ABTS, DPPH, hydrogen peroxide scavenging, metal chelating properties, butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), and α-tocopherol. The results exhibited that methanol, aqueous, and chloroform extracts had high antioxidant power in all tests compared to industrial antioxidants (34).
3.3.4. A. chamaepitys subsp. chia (Ajuga comata Stapf.)
3.3.4.1. Anti-colitis Effect
Ajuga comate Stapf. is the synonym of A. chamaepitys subsp. chia distributed in Golestan, Mazandaran, Guilan, Azerbaijan, Kordestan, Hamedan, and Tehran provinces, Iran (42). In folk medicine of Turkey, it is a plant used for diarrhea. In a study reported by Kupeli Akkol et al., the total methanolic extract (MeOH) and fractions of aerial fragments of A. chamaepitys subsp. chia were examined in a colitis model with acetic acid in rats. The MeOH extract and n-Buthanol fraction controlled the caspase-3, myeloperoxidase, TNF-α, IL-6 levels, and antioxidant factors. Furthermore, ajugoside, asperulosidic acid, and deacetyl-asperulosidic acid were isolated from the n-BuOH fraction (32).
3.3.4.2. Antibacterial Effect
In the study by Asgharian et al., the extracts of n-hexane, dichloromethane, and methanol extracts of aerial parts of the plant were studied against different microorganisms by the agar disk diffusion method. The effective extracts were then fractionated by the VLC technique and determined by the MIC serial dilution method. Finally, Ajuga comata did not show any inhibitory effect against any of the microorganisms S. aureus and B. subtilis (31).
3.3.5. Ajuga orientalis
3.3.5.1. Antibacterial Effect
In the study of Goger et al., the antibacterial effect of a methanolic extract of A. orientalis in the flowering stage was evaluated against seven strains E. coli, S. aureus, S. typhimurium, B. cereus, C. albicans, C. tropicalis, and C. parapsilosis. The minimum inhibitory concentration (MIC) of the strains was assessed by broth microdilution methods compared to standard antimicrobial drugs such as ampicillin, tetracycline, ketoconazole, and oxiconazole. The results showed that A. orientalis extract had an antimicrobial effect against all tested microorganisms and was more effective against the yeast C. tropicalis (MIC = 78.12 μg/mL). Due to its antimicrobial activity, especially against Candida, the extract of this plant can be used for the natural prevention of Candida infections (29).
3.3.5.2. Plant Antifungal Effect
In an in vitro study, the effects of essential oils and extracts of A. orientalis were investigated against Phytophthora capsicileon, a pathogenic fungus causing common root throat burn red pepper disease. The results showed that the chloroform extract of the plant had the highest inhibitory effect (MIC 0.800 to 0.825 mg/mL) on this fungus (29).
3.3.6. Ajuga austroiranica Rech.f.
Ajuga austroiranica is a beautiful perennial plant widely distributed on rocky slopes and crevices in the mountainous regions of southern and southwestern Iran. The analysis of volatile essential oil compounds obtained from A. austroiranica collected from Fars province was performed by GC/MS. The result of the analysis showed that monoterpenes (30.5% pinene structure) were the main components of the essential oil (Table 3) (57).
3.3.7. Ajuga reptans L.
Ajuga reptans are usually known as bugle, bugleweed, and common bugle. Ajuga reptans L. is distributed in the center and especially the eastern part of Europe, northern Iran (Mazandaran and Guilan provinces), and the Caucasus (42). In folk medicine, A. reptans L. is used as antidiabetic, anti-hypertensive, diuretic, and hepatoprotective medicinal plant (77). Several phytochemicals have been identified in A. reptans, such as ecdysteroids, anthocyanidin glycosides, phenylpropanoid glycoside, neo-clerodane diterpenes, and iridoids that possess antioxidant, antimicrobial, and anti-inflammatory activity (43, 78).
3.3.7.1. Antifeedant Effect
Insect antifeedant evaluation of neo-clerodane diterpenoids isolated from the aerial parts of A. reptans showed that 14,15-dehydroajugareptans had a significant effect on sixth stadium larvae of Spodoptera littoralis (41).
3.3.7.2. Anti-colitis Effect
Di Paola et al. reported that teupolioside, a phenylpropanoid glycoside, produced by A. reptans cell line, meaningfully decreased diarrhea manifestations and body weight loss in a rat model of colitis by applying a significant reduction in colonic myeloperoxidase activity and malondialdehyde levels. Moreover, the reduced release of pro-inflammatory cytokines was observed. Therefore, teupolioside may be valuable for treating inflammatory bowel disease (44).
3.3.7.3. Antioxidant and Antibacterial Effect
The antioxidant and antibacterial potential of active compounds from A. reptans flowers were evaluated in a study by Toiu et al. The results indicated that total phenols, flavonoids, anthocyanins, and iridoids were higher in ethanol/water extraction with maceration and reflux heating than methanol/water extraction.
Besides, A. reptans flowers extracts showed different degrees of DPPH radical scavenging activity. The ethanol extract showed the highest radical scavenging effect (49.35 ± 2.91 μg/mL), and also a high antioxidant activity (IC50 ≤ 50 μg/mL).
The antibacterial assay of A. reptans flower extracts showed moderate antibacterial activity against P. aeruginosa, L. monocytogenes, E. coli, and S. typhimurium. In addition, A. reptans ethanol extract showed the most significant antimicrobial effect on S. aureus and P. aeruginosa (43).
4. Conclusions
The aim of this research was to investigate different species of Ajuga growing in Iran in terms of traditional uses, phytochemical compositions, and pharmacological or clinical studies. As known, A. chamaecistus is the most widespread species in Iran with five exclusive subspecies. Also, numerous phytochemical and pharmacological studies have been performed on various species, especially tomentella. Various pharmacological effects have been reported on these species, confirming the traditional use of the genus Ajuga in many cases, such as anti-inflammatory, hypoglycemic, and analgesic activities. Given that most pharmacological studies have been performed on animals, and on the other hand, toxicological studies have been assessed on some species, it has been shown that the plants of this genus are non-toxic, and there is a need for clinical studies on the use of medicinal products containing these plants or phytochemical compounds isolated from them as an analgesic and anti-inflammatory agents. Since different species of these plants have valuable compounds such as phytoecdysteroids, which can be used in cosmetics for wound healing and hair growth, it seems that studies in these fields can be considered therapeutically and economically valuable.
The results of modern medicine were consistent with traditional medicine in many cases, such as analgesic, anti-inflammatory, and hypoglycemic properties. Due to the beneficial therapeutic effects of the Ajuga genus, it can be considered in future clinical studies and the production of complementary drugs. Also, given that preclinical and clinical studies are not yet sufficient, the results of the studies should be carefully interpreted and generalized.
References
-
1.
Küçükbay F, Kuyumcu E, Yildiz B. Essential Oil Composition from the Aerial Parts of Ajuga orientalis L. Growing in Turkey. Asian J Chem. 2013;25(16):9126-8. https://doi.org/10.14233/ajchem.2013.15045.
-
2.
Khanavi M, Davoodipoor AM, Sadati SN, Ardekani MR, Sharifzadeh M. Antinociceptive effect of some extracts from Ajuga chamaecistus Ging. ssp. tomentella (Boiss.) Rech. f. aerial parts. Daru. 2014;22:56. [PubMed ID: 25022284]. [PubMed Central ID: PMC4230404]. https://doi.org/10.1186/2008-2231-22-56.
-
3.
Movahhedin N, Zengin G, Bahadori MB, Sarikurkcu C, Bahadori S, Dinparast L. Ajuga chamaecistus subsp. scoparia (Boiss.) Rech.f.: A new source of phytochemicals for antidiabetic, skin-care, and neuroprotective uses. Ind Crops Prod. 2016;94:89-96. https://doi.org/10.1016/j.indcrop.2016.08.028.
-
4.
Mohammadhosseini M, Pazoki A, Zamani HA, Akhlaghi H. Chemical Composition of the Essential Oil from Aerial Parts ofAjuga chamaecistusGing. subsp. Scopria in Brackish Regions of Iran. J Essent Oil-Bear Plants. 2011;14(1):101-5. https://doi.org/10.1080/0972060x.2011.10643907.
-
5.
İlkay ZA. An anatomical study of medicinal species Ajuga orientalis L. (Lamiaceae) from Turkey. J Med Plant Res. 2014;8(7):331-8. https://doi.org/10.5897/jmpr2013.5336.
-
6.
Yildirim C, Cansaran A, Çali IÖ. Trichome morphology of Ajuga orientalis L. (Lamiaceae) from Turkey. Bangladesh J Bot. 2014;43(1):91-5. https://doi.org/10.3329/bjb.v43i1.19754.
-
7.
DÖNmez Ş, ÖNal F. Germination Ability and Biochemical Properties of Ajuga Chamaepitys Subsp. Chia Var. Chia and Ajuga Orientalis Cultivated in Climatic Conditions in Lake District, Turkey. Appl Ecol Environ Res. 2019;17(2):3837-48. https://doi.org/10.15666/aeer/1702_38373848.
-
8.
Sadati Lamardi SN, Tahroodi S, Khanavi M, Hosseini Doust R. Antibacterial Activity of Aerial Part Extracts and Fractions of Ajuga chamaecistus ssp. tomentella. Trad Integr Med. 2017;2(2):61-6.
-
9.
Haghir Ebrahimabadi A, Noohi M, Shahbazi-Alavi H, Batooli H. Antibacterial properties of Ajuga chamaecistus subsp. scoparia and chemical composition of its oils. J Medicinal Plants By- Products. 2016;5(1):67-73.
-
10.
Sadati Lamardi SN, Majidi Z, Alipour C, Farajzadeh S, Gohari A, Shafaroodi H, et al. Antioxidant potential, hypoglycemic effect and safety of Ajuga chamaecistus Ging. ssp. tomentella (Boiss.) Rech. f. aerial parts. Res J Pharmacogn. 2018;5(4):53-63.
-
11.
Khanavi M, Najafi B, Sadati SN, Abai MR, Vatandoost H. Chemical Constitute and Larvicidal Activity of Fractions of Ajuga chamaecistus tomentella Plant against Malaria Vector Anopheles stephensi. J Arthropod Borne Dis. 2017;11(1):116-23. [PubMed ID: 29026858]. [PubMed Central ID: PMC5629293].
-
12.
Vanyolos A, Simon A, Toth G, Polgar L, Kele Z, Ilku A, et al. C-29 ecdysteroids from Ajuga reptans var. reptans. J Nat Prod. 2009;72(5):929-32. [PubMed ID: 19338317]. https://doi.org/10.1021/np800708g.
-
13.
Castro A, Coll J, Tandron YA, Pant AK, Mathela CS. Phytoecdysteroids from Ajuga macrosperma var. breviflora roots. J Nat Prod. 2008;71(7):1294-6. [PubMed ID: 18529078]. https://doi.org/10.1021/np800131f.
-
14.
Coll J. NMR shift data of neo-clerodane diterpenes from the genus Ajuga. Phytochem Anal. 2002;13(6):372-80. [PubMed ID: 12494759]. https://doi.org/10.1002/pca.671.
-
15.
Manguro LO, Ogur JA, Okora DM, Wagai SO, Lemmen P. Further flavonol and iridoid glycosides from Ajuga remota aerial parts. J Asian Nat Prod Res. 2007;9(6-8):617-29. [PubMed ID: 17943556]. https://doi.org/10.1080/10286020600979480.
-
16.
Bouyahya A, El Omari N, Belmehdi O, Lagrouh F, El Jemli M, Marmouzi I, et al. Pharmacological investigation of Ajuga iva essential oils collected at three phenological stages. Flavour Fragr J. 2020;36(1):75-83. https://doi.org/10.1002/ffj.3618.
-
17.
Naghibi F, Mosadegh M, Ghorbani AB, Mohammadi Motamed S. Labiatae family in folk medicine in iran: From ethnobotany to pharmacology. Iran J Pharm Sci. 2005;4(2):63-79.
-
18.
Jorjani M. [Zakhire Kharazmshahi (Kharazmid reservoir) Qom]. Qom, Iran: Institute of Natural Medicine Resurgence Press; 2012. 180 p. Persian.
-
19.
Bellakhdar J, Claisse R, Fleurentin J, Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoea. J Ethnopharmacol. 1991;35(2):123-43. [PubMed ID: 1809818]. https://doi.org/10.1016/0378-8741(91)90064-k.
-
20.
Cocquyt K, Cos P, Herdewijn P, Maes L, Van den Steen PE, Laekeman G. Ajuga remota Benth.: from ethnopharmacology to phytomedical perspective in the treatment of malaria. Phytomedicine. 2011;18(14):1229-37. [PubMed ID: 22015320]. https://doi.org/10.1016/j.phymed.2011.08.063.
-
21.
Aghili MH. Makhzan-al-Advieh. Qom, Iran: Noor e Way; 2011. Persian.
-
22.
Israili ZH, Lyoussi B. Ethnopharmacology of the plants of genus Ajuga. Pak J Pharm Sci. 2009;22(4).
-
23.
Sadati N, Jenett-Siems K, Siems K, Ardekani MR, Hadjiakhoondi A, Akbarzadeh T, et al. Major constituents and cytotoxic effects of Ajuga chamaecistus ssp. tomentella. Z Naturforsch C J Biosci. 2012;67(5-6):275-81. [PubMed ID: 22888532]. https://doi.org/10.1515/znc-2012-5-606.
-
24.
Aghili Khorasani MH. [Gharabadin e kabir]. Qom, Iran: Noor e Way; 2011.
-
25.
Tonkaboni M. [Tohfeh al-momenin]. Tehran, Iran: Shahid Beheshti University of Medical Sciences; 2007. Persian.
-
26.
Jahan MN. [Exir Azam (Great Panacea)]. Tehran, Iran: Research Institute for Islamic and Complementary Medicine; 2008. Persian.
-
27.
Al-Razi MZ. [Al-Hawi]. Beirut, Lebanon: Dare Ehya al Toras al Arabi; 2004. Arabic.
-
28.
Luan F, Han K, Li M, Zhang T, Liu D, Yu L, et al. Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Species from the Genus Ajuga L.: A Systematic Review. Am J Chin Med. 2019;47(5):959-1003. [PubMed ID: 31416340]. https://doi.org/10.1142/S0192415X19500502.
-
29.
Göger F, Köse YB, Göger G, Demirci F. Phytochemical characterization of phenolics by LC-MS/MS and biological evaluation of Ajuga orientalis from Turkey. Bangladesh J Pharmacol. 2015;10(3):639. https://doi.org/10.3329/bjp.v10i3.23500.
-
30.
Mosaddegh M, Naghibi F, Moazzeni H, Pirani A, Esmaeili S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J Ethnopharmacol. 2012;141(1):80-95. [PubMed ID: 22366675]. https://doi.org/10.1016/j.jep.2012.02.004.
-
31.
Asgharian P, Heshmati Afshar F, Mahmoodzadeh E, Hallaj-Nezhadi S. [Antibacterial Activity of Different Extracts of Aerial Parts of Chondrilla juncea L, Ajuga comata Stapf, Nepeta ucrainica L, and Delphinium speciosum MB]. J Maz Univ Med Sci. 2019;29(180):24-35. Persian.
-
32.
Kupeli Akkol E, Ilhan M, Karpuz B, Tastan H, Sobarzo-Sanchez E, Khan H. Beneficial effects of Ajuga chamaepitys (L.) Schreber subsp. chia (Schreber) and its iridoids on the colitis model: Histopathological and biochemical evidence. Food Chem Toxicol. 2020;144:111589. [PubMed ID: 32726593]. https://doi.org/10.1016/j.fct.2020.111589.
-
33.
Mart S, Türkmen N. A survey on wild plants with ethnobotanical use in the Bahçe and Hasanbeyli districts of Osmaniye, Turkey. GSC Biol Pharm Sci. 2018;5(3):28-35. https://doi.org/10.30574/gscbps.2018.5.3.0133.
-
34.
Turkoglu S, Turkoglu I, Kahyaoglu M, Celık S. Determination of antimicrobial and antioxidant activities of Turkish endemic Ajuga chamaepitys (L.) Schreber subsp. euphratica PH Davis (Lamiaceae). J Med Plant Res. 2010;4(13):1260-8.
-
35.
Venditti A, Frezza C, Maggi F, Lupidi G, Bramucci M, Quassinti L, et al. Phytochemistry, micromorphology and bioactivities of Ajuga chamaepitys (L.) Schreb. (Lamiaceae, Ajugoideae): Two new harpagide derivatives and an unusual iridoid glycosides pattern. Fitoterapia. 2016;113:35-43. [PubMed ID: 27373875]. https://doi.org/10.1016/j.fitote.2016.06.016.
-
36.
Delazar A, Delnavazi M, Yassa N, Parkhideh S, Delazar N, Nahar L, et al. Essential oil composition and isolation of freeradical-scavenging phenolic glycosides from the aerial parts of Ajuga chamaepitys growing in Iran. Rev Bras Farmacog. 2012;22(2):399-5. https://doi.org/10.1590/s0102-695x2011005000215.
-
37.
Azizan J, Fallah-Bagher-Shaidaei H, Kefayati H. Chemical Constituents of the Essential Oil of Ajuga chamaepitys Growing in Iran. J Essen Oil Res. 2011;14(5):344-5. https://doi.org/10.1080/10412905.2002.9699877.
-
38.
Ulukanli Z, Ulukanli S, Ozbay H, Ilcim A, Tuzcu M. Antimicrobial Activities of Some Plants from the Eastern Anatolia Region of Turkey. Pharm Biol. 2005;43(4):334-9. [PubMed ID: 28925834]. https://doi.org/10.1080/13880200590951757.
-
39.
Mohammadi-Bazargani M, Rohloff J, Atapour M. Comparative Analysis of Essential Oil and Different Extracts of Ajuga chamaecistus ssp. scoparia on Antibacterial Activity. J Essent Oil-Bear Plants. 2017;20(4):1117-24. https://doi.org/10.1080/0972060x.2017.1365017.
-
40.
Ardekani M, Khanavi M, Taheri P, Samadi N, Safaripour E, Salimpour F. The Essential Oil Composition ofAjuga chamaecistusGing. subsp.tomentellaRech. f. J Essent Oil-Bear Plants. 2010;13(1):45-51. https://doi.org/10.1080/0972060x.2010.10643789.
-
41.
Bremner PD, Simmonds MS, Blaney WM, Veitch NC. Neo-clerodane diterpenoid insect antifeedants from Ajuga reptans cv catlins giant. Phytochemistry. 1998;47(7):1227-32. https://doi.org/10.1016/s0031-9422(97)00706-1.
-
42.
Mozaffarian V. [Identification of medicinal and aromatic plants of Iran]. Tehran, Iran: Farhange Moaser; 2013. 481 p. Persian.
-
43.
Toiu A, Vlase LAURIAN, Gheldiu AM, Vodnar D, Oniga I. Evaluation of the antioxidant and antibacterial potential of bioactive compounds from Ajuga reptans extracts. Farmacia. 2017;65(3):351-5.
-
44.
Di Paola R, Esposito E, Mazzon E, Riccardi L, Caminiti R, Dal Toso R, et al. Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis. Biochem Pharmacol. 2009;77(5):845-57. [PubMed ID: 19070602]. https://doi.org/10.1016/j.bcp.2008.11.010.
-
45.
de la Torre MC, Rodríguez B, Bruno M, Piozzi F, Vassallo N, Bondí ML, et al. Neo-clerodane diterpenoids from Ajuga australis and A. orientalis. Phytochemistry. 1997;45(1):121-3. https://doi.org/10.1016/s0031-9422(96)00850-3.
-
46.
Chen H, Tan RX, Liu ZL, Zhang Y, Yang L. Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J Nat Prod. 1996;59(7):668-70. [PubMed ID: 8759163]. https://doi.org/10.1021/np960385s.
-
47.
Shimomura H, Sashida Y, Ogawa K. Iridoid glucosides and phenylpropanoid glycosides in Ajuga species of Japan. Phytochemistry. 1987;26(7):1981-3. https://doi.org/10.1016/s0031-9422(00)81742-2.
-
48.
Terahara N, Callebaut A, Ohba R, Nagata T, Ohnishi-Kameyama M, Suzuki M. Acylated anthocyanidin 3-sophoroside-5-glucosides from Ajuga reptans flowers and the corresponding cell cultures. Phytochemistry. 2001;58(3):493-500. [PubMed ID: 11557083]. https://doi.org/10.1016/s0031-9422(01)00172-8.
-
49.
Akbay P, Calis I, Heilmann J, Sticher O. Ionone, iridoid and phenylethanoid glycosides from Ajuga salicifolia. Z Naturforsch C J Biosci. 2003;58(3-4):177-80. [PubMed ID: 12710724]. https://doi.org/10.1515/znc-2003-3-406.
-
50.
Castro A, Coll J, Arfan M. Neo-clerodane diterpenoids from Ajuga bracteosa. J Nat Prod. 2011;74(5):1036-41. [PubMed ID: 21539300]. https://doi.org/10.1021/np100929u.
-
51.
Qing X, Yan H, Ni Z, Vavricka CJ, Zhang M, Shi Q, et al. Chemical and pharmacological research on the plants from genus Ajuga. Heterocycl Comm. 2017;23(4). https://doi.org/10.1515/hc-2017-0064.
-
52.
Jaffal SM, Abbas MA, Alsalem M, Al-Najjar BO. Evidence for the involvement of opioid receptor in Ajuga chamaepitys action in chemical and thermal models of pain in BALB/c mice. Medicinal Chem Res. 2019;28(7):992-9. https://doi.org/10.1007/s00044-019-02353-1.
-
53.
Boneva IM, Mikhova BP, Malakov PY, Papanov GY, Duddeck H, Spassov SL. Neo-clerodane diterpenoids from Ajuga chamaepitys. Phytochemistry. 1990;29(9):2931-3. https://doi.org/10.1016/0031-9422(90)87108-7.
-
54.
Ono M, Furusawa C, Ozono T, Oda K, Yasuda S, Okawa M, et al. Four new iridoid glucosides from Ajuga reptans. Chem Pharm Bull (Tokyo). 2011;59(8):1065-8. [PubMed ID: 21804257]. https://doi.org/10.1248/cpb.59.1065.
-
55.
Coll J, Tandrón YA. neo-Clerodane diterpenoids from Ajuga: structural elucidation and biological activity. Phytochemistry Reviews. 2007;7(1):25-49. https://doi.org/10.1007/s11101-006-9023-3.
-
56.
Sajjadi SE, Ghannadi A, Sajjadi SE, Ghannadi A. Volatile oil composition of the aerial parts of Ajuga orientalis L. from Iran. Z Naturforsch C J Biosci. 2004;59(3-4):166-8. [PubMed ID: 15241917]. https://doi.org/10.1515/znc-2004-3-404.
-
57.
Javidnia K, Miri R, Soltani M, Khosravi AR. Chemical Constituents of the Essential Oil ofAjuga austro-iranicaRech. f. (Lamiaceae) from Iran. J Essent Oil Res. 2010;22(5):392-4. https://doi.org/10.1080/10412905.2010.9700354.
-
58.
Morteza-Semnani K, Azadbakht M, Hooshmand A. Chemical Composition of the Essential Oils from the Flowers and Leaves of Ajuga reptans. Chem Nat Compd. 2018;54(2):375-6. https://doi.org/10.1007/s10600-018-2352-9.
-
59.
Frezza C, Venditti A, Pizzoli F, Serafini I, Ciccòla A, Pitorri M, et al. Essential oil composition and total metabolite content of a chemotype of Ajuga reptans L. (Lamiaceae) collected in Central Italy. Plant Biosyst. 2018;153(4):552-8. https://doi.org/10.1080/11263504.2018.1515121.
-
60.
Guo P, Li Y, Xu J, Guo Y, Jin DQ, Gao J, et al. neo-Clerodane diterpenes from Ajuga ciliata Bunge and their neuroprotective activities. Fitoterapia. 2011;82(7):1123-7. [PubMed ID: 21807075]. https://doi.org/10.1016/j.fitote.2011.07.010.
-
61.
Chiou CT, Kuo YH, Chan YY, Juang SH, Chan HH, Wu TS. Ajugalide-B (ATMA) is an anoikis-inducing agent from Ajuga taiwanensis with antiproliferative activity against tumor cells in vitro. Phytochemistry. 2012;80:64-9. [PubMed ID: 22633845]. https://doi.org/10.1016/j.phytochem.2012.05.005.
-
62.
Dong B, Yang X, Liu W, An L, Zhang X, Tuerhong M, et al. Anti-inflammatory neo-Clerodane Diterpenoids from Ajuga pantantha. J Nat Prod. 2020;83(4):894-904. [PubMed ID: 32216313]. https://doi.org/10.1021/acs.jnatprod.9b00629.
-
63.
Liu W, Song Z, Wang H, Yang X, Joubert E, Zhang J, et al. Diterpenoids as potential anti-inflammatory agents from Ajuga pantantha. Bioorg Chem. 2020;101:103966. [PubMed ID: 32506016]. https://doi.org/10.1016/j.bioorg.2020.103966.
-
64.
Funes M, Garro MF, Tosso RD, Maria AO, Saad JR, Enriz RD. Antinociceptive effect of neo-clerodane diterpenes obtained from Baccharis flabellata. Fitoterapia. 2018;130:94-9. [PubMed ID: 30145332]. https://doi.org/10.1016/j.fitote.2018.08.017.
-
65.
Guerrero MF, Puebla P, Carron R, Martin ML, San Roman L. Vasorelaxant effect of new neo-clerodane diterpenoids isolated from Croton schiedeanus. J Ethnopharmacol. 2004;94(1):185-9. [PubMed ID: 15261981]. https://doi.org/10.1016/j.jep.2004.05.018.
-
66.
Sun Z, Li Y, Jin DQ, Guo P, Song H, Xu J, et al. neo-Clerodane diterpenes from Ajuga decumbens and their inhibitory activities on LPS-induced NO production. Fitoterapia. 2012;83(8):1409-14. [PubMed ID: 22917649]. https://doi.org/10.1016/j.fitote.2012.08.003.
-
67.
Wang H, Teng X, Zhang Y, Gu Q, He L. Diterpenoids from the Whole Plants of Ajuga nipponensis and Their Inhibition of RANKL-Induced Osteoclastogenesis. Chem Biodivers. 2021;18(1). e2000780. [PubMed ID: 33205900]. https://doi.org/10.1002/cbdv.202000780.
-
68.
Tundis R, Loizzo MR, Menichini F, Statti GA, Menichini F. Biological and pharmacological activities of iridoids: recent developments. Mini Rev Med Chem. 2008;8(4):399-420. [PubMed ID: 18473930]. https://doi.org/10.2174/138955708783955926.
-
69.
Takasaki M, Tokuda H, Nishino H, Konoshima T. Cancer chemopreventive agents (antitumor-promoters) from Ajuga decumbens. J Nat Prod. 1999;62(7):972-5. [PubMed ID: 10425119]. https://doi.org/10.1021/np990033w.
-
70.
Atay I, Kirmizibekmez H, Kaiser M, Akaydin G, Yesilada E, Tasdemir D. Evaluation of in vitro antiprotozoal activity of Ajuga laxmannii and its secondary metabolites. Pharm Biol. 2016;54(9):1808-14. [PubMed ID: 26734766]. https://doi.org/10.3109/13880209.2015.1129542.
-
71.
Gautam R, Jachak SM, Saklani A. Anti-inflammatory effect of Ajuga bracteosa Wall Ex Benth. mediated through cyclooxygenase (COX) inhibition. J Ethnopharmacol. 2011;133(2):928-30. [PubMed ID: 21073945]. https://doi.org/10.1016/j.jep.2010.11.003.
-
72.
Dinan L. The Karlson Lecture. Phytoecdysteroids: what use are they? Arch Insect Biochem Physiol. 2009;72(3):126-41. [PubMed ID: 19771554]. https://doi.org/10.1002/arch.20334.
-
73.
Hamden K, Ayadi F, Jamoussi K, Masmoudi H, Elfeki A. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors. 2008;33(3):165-75. [PubMed ID: 19478420]. https://doi.org/10.1002/biof.5520330302.
-
74.
Cai YJ, Dai JQ, Fang JG, Ma LP, Hou LF, Yang L, et al. Antioxidative and free radical scavenging effects of ecdysteroids from Serratula strangulata. Can J Physiol Pharmacol. 2002;80(12):1187-94. [PubMed ID: 12564645]. https://doi.org/10.1139/y02-152.
-
75.
Ramazanov N. Phytoecdysteroids and Other Biologically Active Compounds from Plants of the Genus Ajuga. Chem Nat Compd. 2005;41(4):361-9. https://doi.org/10.1007/s10600-005-0153-4.
-
76.
Kazemi Saeed F, Jamzad Z, Vaziri A, Jalil A, Sefidkon F. Foliar anatomical studies of Ajuga chamaecistus (lamiaceae) from iran. Iran J Bot. 2019;25(2):152-81.
-
77.
Esposito T, Sansone F, Auriemma G, Franceschelli S, Pecoraro M, Picerno P, et al. Study on Ajuga reptans Extract: A Natural Antioxidant in Microencapsulated Powder Form as an Active Ingredient for Nutraceutical or Pharmaceutical Purposes. Pharmaceutics. 2020;12(7). [PubMed ID: 32708873]. [PubMed Central ID: PMC7407557]. https://doi.org/10.3390/pharmaceutics12070671.
-
78.
Toiu A, Mocan A, Vlase L, Parvu AE, Vodnar DC, Gheldiu AM, et al. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and In Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules. 2019;24(8). [PubMed ID: 31018502]. [PubMed Central ID: PMC6515068]. https://doi.org/10.3390/molecules24081597.