Abstract
Background:
Human myeloid leukemia is among the hematological cancers associated with arrested differentiation and continued proliferation in promyelocytes. Despite the widespread use of Rheum turkestanicum, as a medicinal plant, there is still a lack of information on its toxicity profile. In this research, the cytotoxic effect of hydroalcoholic extract of R. turkestanicum root was evaluated on leukemic cells.Objectives:
We aimed to assess cellular oxidants, cytotoxicity, apoptosis, and differentiation caused by R. turkestanicum (60 - 500 µg/mL) and arsenic trioxide (50 µM) in leukemic and polymorph nuclear cells during 72 hours.Methods:
In order to determine cell viability, resazurin assay was carried out at 24, 48, and 72 hours following R. turkestanicum treatment (range, 60 - 500 µg/mL). Carboxy 2’, 7’-dichlorofluorescein diacetate was used to examine intracellular reactive oxygen species (ROS) via fluorimetry. For the analysis of apoptotic cells, propidium iodide (PI) flow cytometric assay was performed. Differentiation of cells was evaluated by Giemsa staining and nitro blue tetrazolium reduction.Results:
R. turkestanicum inhibited viability and increased apoptosis of leukemic cells similar to the As2O3 group. R. turkestanicum did not induce differentiation of leukemic cells towards a granulocytic pattern. Based on the findings, no toxic effects were induced by the extract on polymorphonuclear cells.Conclusions:
The present study demonstrated that R. turkestanicum caused apoptosis dose- and time-dependently without causing any differential effects on leukemic cells. The precise signaling pathway by which R. turkestanicum induces apoptosis needs further research.Keywords
1. Background
Acute promyelocytic leukemia (APL) is one of the most threatening hematological malignant cancers (1). Promyelocytes exhibit a failure of myeloid differentiation. Arsenic trioxide (As2O3) is used clinically in the treatment of APL. Apoptosis induction, growth inhibition, and promotion of differentiation constitute the major anticancer mechanism. High doses of As2O3 causes various side effects and toxicity; however replacement or combination of this drug with other anti-cancer compounds can reduce the dose and side effects of As2O3 (2-4).
Development of combination treatments require lower concentrations of As2O3 can be favorable for APL patients (5). Therefore, the search for novel anti-cancer agents is currently receiving great attention. Natural products, especially medicinal plants, have been recognized for as long as potential sources for anti-cancer drugs (6, 7). For example, paclitaxel is used to treat various malignant diseases such as lung and breast cancers and was discovered by isolating the compound from the Pacific yew tree, Taxusbrevifolia (8-10). Therefore, the search for potent anticancer drugs, isolated from plants, is in progress. The recent studies have shown that some medicinal plants such as Spirulina platensis, pomegranate fruit, Cuscuta campestris, Ferula gummosa gum, Epigallocatechin-3-gallate, and Kaempferol have cytotoxic effects on leukemia cells (11-16).
Traditional Chinese medicine has suggested Rheum species, belonging to the Polygonaceae family, for the treatment of different diseases. Anthraquinones, glycosides, and tannins are among the major active components of the Rheum species (17). In addition, emodin, rhein, chrysophanol, physcion, alizarin, and citreorosein are among the anthraquinone derivatives (18).
In recent years, researchers have paid great attention to the antitumor activity of medicinal plants. According to the literature, R. emodi exerts cytotoxic effects on P388 leukemia in mice, as well as human breast carcinoma and liver carcinoma cell lines in humans (19). Also, R. officinale Baill, showing cytotoxicity against A549 and MCF-7, is prescribed for cancer patients (20).
Previous studies have shown the anticancer properties of R. undulatum L. in vivo and in vitro (21). According to various studies, emodin, aloe-emodin, and rhein have antiproliferative properties in cell lines of different cancers (breast cancer with HER-2/neu overexpression, leukemia, hepatoma, human myeloma, and lung carcinoma) and are associated with apoptosis (22-25).
R. turkestanicum, which belongs to the Polygonaceae family, grows extensively in Northeast Iran. The roots of this plant have been traditionally used as antidiabetic, antihypertensive, and anticancer agents. In this regard, Shiezadeh et al. demonstrated the cytotoxic and apoptotic properties of R. turkestanicum in HeLa and MCF-7 cells, without causing toxicity in normal cell lines (26).
2. Objectives
Despite recommendations in traditional medicine, limited research has been performed on R. turkestanicum as an anticancer plant. Accordingly, we aimed to assess the cytotoxic effects and differentiation of R. turkestanicum in leukemic cells (HL60 and NB4) in comparison with arsenic trioxide (as the positive control).
3. Methods
3.1. Plant Materials
R. turkestanicum roots were gathered from Chenar village, Razavi Khorasan, Iran. Experts at the Herbarium Department of Ferdowsi University of Mashhad identified the plant (No., 21377). The dried root (60 g) was ground into fine powder; then, 50 g of the powder was extracted in a Soxhlet system using 70% ethanol during 48 hours. The hydroalcoholic extract was dried on a water bath and kept frozen (-20°C) for further use (yield, 19% w/w). Phosphate-buffered saline (PBS) was used to dissolve the extract to a final dose of 50 mg/mL before further use in cytotoxicity and apoptosis assays.
3.2. Cell Lines and Reagents
Pasteur Institute of Iran provided HL60 and NB4 cells. Resazurin reagent (300 μM of resazurin, 78 μM of methylene blue, 1 mM of potassium hexacyanoferrate III, and 1 mM of potassium hexacyanoferrate II), 2,7-dichlorofluorescein diacetate (DCFH-DA), propidium iodide (PI), nitro blue tetrazolium (NBT), phorbol myristate acetate (PMA), and arsenic trioxide (stock concentration of 1 mM) were provided by Sigma Co. (St. Louis, MO, USA). In addition, Gibco BRL (NY, USA) provided high-glucose RPMI 1640 medium, fetal bovine serum (FBS), and penicillin-streptomycin solution. Giemsa stain was purchased from Merck (Darmstadt, Germany).
3.3. Human Normal Cell Isolation and Cell Culture
Polymorphonuclear cells (PMNs) from healthy volunteers were isolated under sterile conditions by two consecutive Ficoll-Hypaque (Pharmacia) density gradient centrifugations (21). PMN was purified to more than 97%, based on the morphological assessment of Wright-stained smears. After rinsing with sterile PBS, RPMI medium was used to resuspend the cells. HL60, NB4, and PMN cells were stored at a temperature of 37°C in a 90% humidified atmosphere (5% CO2).
RPMI 1640 medium was supplemented with 10% FBS, penicillin (100 units/mL), and streptomycin (100 µg/mL). Afterwards, incubation of cells was performed with various doses of R. turkestanicum (60 - 500 µg/mL) and As2O3 (50 µM) for 24, 48, and 72 hours, respectively. For every dose and time interval, we used an untreated control, receiving 0.5% PBS medium (an equal volume). All treatments were performed in triplicate.
3.4. Cell Viability
A resazurin reagent was used for measuring cell viability. To each well of the culture plates (96-well), 100 µL of NB4, HL60, and PMN cells (1 × 105 cells) was added. R. turkestanicum extract (60 - 500 µg/mL) or As2O3 (50 µM) was used to treat the cells for 24, 48, and 72 hours. Afterwards, 20 µL of the resazurin reagent was added to each well, and incubation was performed during four hours. The intensity of fluorescence emission from the resorufin product was examined (relative to the viable cell count in each well), using a fluorescence Victor X5 2030 Multilabel Plate Reader (PerkinElmer, Shelton, Connecticut) at excitation/emission wavelengths of 485 nm/530 nm (27).
3.5. ROS Assessment
In brief, HL60 and NB4 cells (105/well) were incubated with DCF-DA (20 µM) for 30 minutes in the dark and then treated with R. turkestanicum extract (60 - 500 µg/mL) or As2O3 (50 µM) for two hours. Then, the cells were transferred to a 75-mm Falcon™ polystyrene tube. After rinsing twice with PBS, a FLUOstar Galaxy fluorescence plate reader (PerkinElmer 2030, Finland) was used to determine DCF fluorescence intensity at excitation/emission wavelengths of 485 nm/530 nm (28).
3.6. Apoptosis
PI staining of the treated cells was performed to detect the apoptotic cells; then, flow cytometry was applied to determine the sub-G1 peak. In short, R. turkestanicum extract (125 - 500 µg/mL) or As2O3 (50 µM) was used to treat HL60 and NB4 cells during 48 hours. Incubation of cells was performed at 4°C in darkness overnight, using 500 µL of hypotonic PI buffer (50 µg/mL). Afterwards, flow cytometry was applied to examine the samples. Overall, 10,000 events were reported per sample, and WINMDI software was used to assess the data (10).
3.7. Morphological and Differentiation Assay
Diagnosis and morphological typing were determined by standard techniques using Giemsa-stained smears, and differentiation test was performed for NBT reduction. The NBT reaction acts as a sensitive and simply quantified marker for differentiation of leukemic cells; moreover, it can eliminate observer bias caused by morphological evaluation alone.
The cells (1 × 106 cells/mL) were treated in a 6-well plate with R. turkestanicum extract (60, 125 µg/mL) or As2O3 (0.5 µM) (29) for three days. Then, PBS was used to wash the cells twice, and the pellets were resuspended in 0.2% NBT (with an equal volume) and dissolved in Dulbecco’s phosphate-buffered saline (consisting of 200 ng/mL of PMA freshly diluted). After 25 minutes of incubation at a temperature of 37°C in darkness, cytospin was prepared on the slides, and Giemsa staining was carried out; then, presence of blueblack formazan granules was assessed in 300 cells (30).
3.8. Statistics
GraphPad Prism version 6 (GraphPad Software Inc., CA, USA) was used for analyzing the data. ANOVA test as well as Tukey’s multiple comparison test were applied for comparison among the groups. The significance level was set at 0.05. In this study, the data are presented as mean ± SEM of three independent analyses, which were performed in triplicate.
4. Results
4.1. Dose-Dependent Reduction of Cell Viability Using R. turkestanicum
To evaluate the toxic effects of R. turkestanicum, HL60 and NB4 cells were incubated with different doses of R. turkestanicum (60 - 500 µg/mL) and As2O3 (50 µM, as the positive control) over 72 hours; the resazurin reagent was used to analyze cell viability (Figure 1). The PMN cells were incubated with R. turkestanicum (200 - 800 µg/mL) for 24 hours. In comparison with PMN cells, R. turkestanicum-mediated toxicity was significantly higher in HL60 and NB4 cells. The findings are graphically presented in Figure 1 and are summarized in Table 1.
IC50 (Concentration of 50% Inhibition) Values of R. Turkestanicum in NB4 and HL60 Cells During 24 - 72 Hours
| Treatment | Cell Lines, h | |||||
|---|---|---|---|---|---|---|
| HL60 Cells | NB4 Cells | |||||
| 24 | 48 | 72 | 24 | 48 | 72 | |
| R. turkestanicum, µg/mL | 518.60 | 366.20 | 99.02 | 597.80 | 506.00 | 460.20 |
Anti-proliferative effects of R. turkestanicum on leukemic cells. HL60 andNB4 cells were treated with different concentrations of R. turkestanicum (60 - 500 µg/mL) or As2O3 (50µM) for 24 - 72 hours. Normal polymorph nuclear (PMN) cells were also treated with R. turkestanicum (200 - 800 µg/mL) for 24 hours. The percentage cell viability (quantitated by resazurin assay) was normalized against the negative controls for each cell type. Data are expressed as mean ± SEM of 3 separate experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 as compared with the control value.

As shown in Figure 1, treatment with R. turkestanicum could reduce cell viability in both cell lines dose- and time-dependently. The IC50 values of R. turkestanicum in HL60 cells were respectively 518.6, 336.2, and 99.0 µg/mL at 24, 48, and 72 hours following treatment. However, at 24, 48, and 72 hours following treatment, IC50 of R. turkestanicumin NB4 cells was found to be 597.8, 506.0, and 460.2 µg/mL, respectively (Table 1).
Treatment of HL60 cells with 250 and 500 µg/mL of R. turkestanicum for 72 hours could decrease cell viability more than As2O3 (P < 0.001). The viability of R. turkestanicum treated HL60 cells at 250 and 500 µg/mL significantly reduced to 15% and 12.1%, respectively versus 30.9% in the As2O3 group (in comparison with the vehicle-treated cells; Figure 1; P < 0.001). On the other hand, viability of R. turkestanicum-treated NB4 cells at 500 µg/mL significantly reduced to 44.4% versus 50.7% in the As2O3 group (50 µM) at 72 hours following treatment (Figure 1, P < 0.001). In contrast, the extract did not have any cytotoxic effects up to 800 µg/mL in PMN cells after 24 hours (Figure 1).
4.2. R. turkestanicum Dose-Dependent Increase in ROS Generation
Our findings showed a regular time- and dose-dependent rise in the ROS generation in HL60 and NB4 cells at two hours after treatment with R. turkestanicum, compared to the control group (Figure 2). On comparing with control HL60 cells, 250 and 500 µg/mL R. turkestanicum induced the formation of ROS during three hours of incubation (165 ± 1.1% and 172 ± 4.2% vs. 147 ± 4.1% in the As2O3 group; P < 0.001 in comparison with the controls). The ROS content majorly increased in NB4 cells two hours after treatment with 500 µg/mL R. turkestanicum, compared to the control group (148 ± 7.2% in the experimental group versus 140 ± 8.1% in the As2O3 group; P < 0.001).
Effect of R. turkestanicum on intracellular reactive oxygen species (ROS) in leukemic cells. NB4 and HL60 cells were treated with different concentrations of R. turkestanicum (60 - 500 µg/mL) or As2O3 (50 µM) for two hours. Data are expressed as mea ± SEM of three independent experiments. **P < 0.01 and ***P < 0.001 as compared with control value.

4.3. R. turkestanicum Concentration-Dependent Induction of Apoptotic Cell Death
Figure 3 shows that R. turkestanicum at high concentrations induces apoptosis in NB4 and HL60 cells, significantly. After 48 hours of treatment, R. turkestanicum treated HL60 cells showed 63.2% (250 µg/mL) and 65.9% (500 µg/mL) against 55.5% in the As2O3 group (50 µM) (P < 0.001 in comparison with the controls). In addition, 48.0% of R. turkestanicum-treated NB4 cells underwent apoptosis, versus 45.5% of cells in the As2O3 group (P < 0.001 in comparison with the vehicle-treated cells). The apoptotic cell count increased concomitantly with concentration in the experimental group. The extract increased apoptosis in HL60 more than NB4 cells, especially at low concentrations (P < 0.001, Figure 3).
Effect of R. turkestanicum on apoptosis in leukemic cells. A, NB4 and HL60 cells were incubated with different concentrations of R. turkestanicum (125 - 500 µg/mL) or As2O3 (50 µM) for 48 hours. Apoptosis was assayed by PI staining and analyzed by flow cytometry. B, Apoptosis rate is shown by bar graph. The data are mean ± SEM of three independent experiments. ***P < 0.001 as compared with the control value.

4.4. R. turkestanicum Did Not Induce Differentiation of Leukemic Cells Toward Granulocyte Pattern
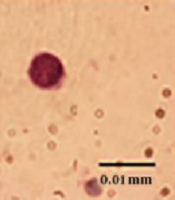
The Giemsa and NBT tests showed that R. turkestanicum did not induce maturation of cells to neutrophils. In the morphological Giemsa assay, the cells showed promyelocytes characteristic with cytoplasmic granules, like control cells. In functional NBT assay, cells did not have any intracellular blue-black formazan deposits (Figure 4).
Effect of R. turkestanicum on morphological and functional properties of granulocyte in leukemic cells. NB4 cells were treated with R. turkestanicum (60 µM) or As2O3 (0.5 µM) for 72 hours. Morphological and functional properties of granulocyte were determined by Gimsa and NBT assays, respectively. In Giemsa-stained slides, As2O3-treated cells (as positive control) showed polymorphonuclear morphology of granulocyte, and R. turkestanicum-treated cells showed promyelocytes characteristic, same as the control. On NBT slides, As2O3-treated cells (as the positive control) showed intracellular blue-black formazan deposits, and R. turkestanicum-treated cells did not have any intracellular blue-black formazan deposits (bar represents 0.01 mm).

5. Discussion
Medicinal plants have been used for the management of different diseases since long ago (23). Medicinal plants and their derivatives have been extensively used in different cultures. Today, use of plants as natural alternatives has grown in popularity (31). Natural products and plant derivatives (including antibiotics) are the major components of various anticancer agents. Consequently, there has been a major focus on natural products as important sources for effective anticancer drugs (32).
The present study is the first to assess the antineoplastic effects of R. turkestanicum in leukemic cells. We report that R. turkestanicum exhibits significant cytotoxicity in cancer cells at selective concentrations (> 125 µg/mL). The cytotoxic effect of R. turkestanicum was more pronounced against the neoplastic cells than human PMN cells. These effects were analogous to those of As2O3, as the standard antileukemic drug.
Based on the present findings, R. turkestanicum leads to dose-dependent inhibition of proliferation and ROS-dependent apoptosis. The results also showed that apoptosis was induced more in HL-60 (AML M2) cells than NB4 (AML M3) cells. This finding might be related to the inherent metabolic features of cancer cells. Previous studies indicated that NB4 cells have highly “glycolytic” properties (33, 34).
Various studies have been performed on the effects of Rheum species on cancer cells. In this study, the cytotoxic effect of R. turkestanicum was evaluated on leukemia cell lines. The evaluated doses did not show similar inhibitory effects in normal cells in the evaluated tumor cell lines. R. turkestanicum not only increased the level of ROS, however, it also induced concomitant increase of apoptosis. Therefore, the cytotoxic effect may be associated with the increase in ROS and apoptotic induction.
The major active ingredients of Rheum species include anthraquinones, dianthrones, glycosides, and tannins. In addition, emodin, rhein, chrysophanol, physcion, alizarin, and citreorosein are among the anthraquinone derivatives (18). According to recent research, physcion prevents the development of different human cancer cell lines such as HeLa (cervical cancer), A549 (lung cancer), HL-60 (leukemia), and SW680 (colon cancer) (35).
Emodin, as an active compound of Rheum species has anticancer effects. It could induce differentiation of APL cells. Moreover, by inhibiting the PI3K/Akt pathway, it could induce AML apoptosis both dose- and time-dependently. The cytotoxicity of the extract against leukemia cells could be mediated by active compounds such as emodin or physicon (23).
In conclusion, the current findings showed that R. turkestanicum could inhibit the growth and proliferation of leukemic cells and induce apoptosis via increased ROS generation, without causing any differential effects in leukemic cells. The major difference in cytotoxicity between cancer and normal cells is suggestive of the great potential of R. turkestanicum as a new option in cancer treatment. The IC50 values reported in our study may be considerably lower if the pharmacological active compounds become pure. The precise signaling pathway, by which R. turkestanicum induce apoptosis, needs further research.
References
-
1.
Intragumtornchai T, Prayoonwiwat W, Swasdikul D, Suwanwela N, Chaimongkol B, Jootar S, et al. Myelodysplastic syndromes in Thailand: a retrospective pathologic and clinical analysis of 117 cases. Leuk Res. 1998;22(5):453-60. [PubMed ID: 9652732]. https://doi.org/10.1016/s0145-2126(98)00022-8.
-
2.
Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13(2):143-6. [PubMed ID: 20187669].
-
3.
Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20(3):556-63. [PubMed ID: 19073863]. https://doi.org/10.1093/annonc/mdn642.
-
4.
Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15(5):489-538. [PubMed ID: 24731404]. https://doi.org/10.1016/S1470-2045(14)70029-4.
-
5.
Peng H, Wang H, Xue P, Hou Y, Dong J, Zhou T, et al. Suppression of NRF2-ARE activity sensitizes chemotherapeutic agent-induced cytotoxicity in human acute monocytic leukemia cells. Toxicol Appl Pharmacol. 2016;292:1-7. [PubMed ID: 26708503]. https://doi.org/10.1016/j.taap.2015.12.008.
-
6.
Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna J Phytomed. 2015;5(2):84-97. [PubMed ID: 25949949]. [PubMed Central ID: PMC4418057].
-
7.
Albayrak S, Aksoy A, Sagdic O, Albayrak S. Antioxidant and antimicrobial activities of different extracts of some medicinal herbs consumed as tea and spices in Turkey. J Food Biochem. 2012;36(5):547-54. https://doi.org/10.1111/j.1745-4514.2011.00568.x.
-
8.
Lee KH. Novel antitumor agents from higher plants. Med Res Rev. 1999;19(6):569-96. [PubMed ID: 10557371].
-
9.
Hadfield JA, Ducki S, Hirst N, McGown AT. Tubulin and microtubules as targets for anticancer drugs. Prog Cell Cycle Res. 2003;5:309-25. [PubMed ID: 14593726].
-
10.
Sadeghnia HR, Ghorbani Hesari T, Mortazavian SM, Mousavi SH, Tayarani-Najaran Z, Ghorbani A. Viola tricolor induces apoptosis in cancer cells and exhibits antiangiogenic activity on chicken chorioallantoic membrane. Biomed Res Int. 2014;2014:625792. [PubMed ID: 25243166]. [PubMed Central ID: PMC4163403]. https://doi.org/10.1155/2014/625792.
-
11.
Moradzadeh M, Hosseini A, Rakhshandeh H, Aghaei A, Sadeghnia HR. Cuscuta campestris induces apoptosis by increasing reactive oxygen species generation in human leukemic cells. Avicenna J Phytomed. 2018;8(3):237-45. [PubMed ID: 29881709]. [PubMed Central ID: PMC5987438].
-
12.
Moradzadeh M, Sadeghnia HR, Mousavi SH, Mahmoodi M, Hosseini A. Ferula gummosa gum induces apoptosis via ROS mechanism in human leukemic cells. Cell Mol Biol (Noisy-le-grand). 2017;63(11):17-22. [PubMed ID: 29208170]. https://doi.org/10.14715/cmb/2017.63.11.4.
-
13.
Moradzadeh M, Tabarraei A, Sadeghnia HR, Ghorbani A, Mohamadkhani A, Erfanian S, et al. Kaempferol increases apoptosis in human acute promyelocytic leukemia cells and inhibits multidrug resistance genes. J Cell Biochem. 2018;119(2):2288-97. [PubMed ID: 28865123]. https://doi.org/10.1002/jcb.26391.
-
14.
Moradzadeh M, Roustazadeh A, Tabarraei A, Erfanian S, Sahebkar A. Epigallocatechin-3-gallate enhances differentiation of acute promyelocytic leukemia cells via inhibition of PML-RARalpha and HDAC1. Phytother Res. 2018;32(3):471-9. [PubMed ID: 29193405]. https://doi.org/10.1002/ptr.5990.
-
15.
Moradzadeh M, Hosseini A, Erfanian S, Rezaei H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol Rep. 2017;69(5):924-8. [PubMed ID: 28646740]. https://doi.org/10.1016/j.pharep.2017.04.008.
-
16.
Kashafi E, Moradzadeh M, Mohamadkhani A, Erfanian S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed Pharmacother. 2017;89:573-7. [PubMed ID: 28258039]. https://doi.org/10.1016/j.biopha.2017.02.061.
-
17.
Hosseini A, Mollazadeh H, Amiri MS, Sadeghnia HR, Ghorbani A. Effects of a standardized extract of Rheum turkestanicum Janischew root on diabetic changes in the kidney, liver and heart of streptozotocin-induced diabetic rats. Biomed Pharmacother. 2017;86:605-11. [PubMed ID: 28027536]. https://doi.org/10.1016/j.biopha.2016.12.059.
-
18.
Dorsey JF, Kao GD. Aloe(-emodin) for cancer? More than just a comforting salve. Cancer Biol Ther. 2007;6(1):89-90. [PubMed ID: 17297301]. https://doi.org/10.4161/cbt.6.1.3845.
-
19.
Rajkumar V, Guha G, Ashok Kumar R. Antioxidant and anti-cancer potentials of rheum emodi rhizome extracts. Evid Based Complement Alternat Med. 2011;2011:697986. [PubMed ID: 21792364]. [PubMed Central ID: PMC3137233]. https://doi.org/10.1093/ecam/neq048.
-
20.
Li WY, Chan SW, Guo DJ, Chung MK, Leung TY, Yu PH. Water extract of Rheum officinale Baill. induces apoptosis in human lung adenocarcinoma A549 and human breast cancer MCF-7 cell lines. J Ethnopharmacol. 2009;124(2):251-6. [PubMed ID: 19397973]. https://doi.org/10.1016/j.jep.2009.04.030.
-
21.
Choi ES, Cho SD, Jeon JG, Cho NP. The apoptotic effect of the hexane extract of rheum undulatum l. In oral cancer cells through the down-regulation of specificity protein 1 and survivin. Lab Anim Res. 2011;27(1):19-24. [PubMed ID: 21826155]. [PubMed Central ID: PMC3145981]. https://doi.org/10.5625/lar.2011.27.1.19.
-
22.
Fazly Bazzaz BS, Khajehkaramadin M, Shokooheizadeh HR. In vitro antibacterial activity of Rheum ribes extract obtained from various plant parts against clinical isolates of Gram-negative pathogens. Iran J Pharm Res. 2010;2:87-91.
-
23.
Chang YC, Lai TY, Yu CS, Chen HY, Yang JS, Chueh FS, et al. Emodin induces apoptotic death in murine myelomonocytic leukemia WEHI-3 cells in vitro and enhances phagocytosis in leukemia mice in vivo. Evid Based Complement Alternat Med. 2011;2011:523596. [PubMed ID: 21660305]. [PubMed Central ID: PMC3108103]. https://doi.org/10.1155/2011/523596.
-
24.
Lee HZ. Effects and mechanisms of emodin on cell death in human lung squamous cell carcinoma. Br J Pharmacol. 2001;134(1):11-20. [PubMed ID: 11522592]. [PubMed Central ID: PMC1572914]. https://doi.org/10.1038/sj.bjp.0704205.
-
25.
Ge X, Luo X, Chen Y, Li M, Jiang S, Wang X. Rhein induces apoptosis of HCT-116 human colon cancer cells via activation of the intrinsic apoptotic pathway. Afr J Biotechnol. 2011;10(61):13244-51. https://doi.org/10.5897/AJB11.1323.
-
26.
Shiezadeh F, Mousavi SH, Amiri MS, Iranshahi M, Tayarani-Najaran Z, Karimi G. Cytotoxic and apoptotic potential of rheum turkestanicum janisch root extract on human cancer and normal cells. Iran J Pharm Res. 2013;12(4):811-9. [PubMed ID: 24523761]. [PubMed Central ID: PMC3920695].
-
27.
Mashkani B, Tanipour MH, Saadatmandzadeh M, Ashman LK, Griffith R. FMS-like tyrosine kinase 3 (FLT3) inhibitors: Molecular docking and experimental studies. Eur J Pharmacol. 2016;776:156-66. [PubMed ID: 26896780]. https://doi.org/10.1016/j.ejphar.2016.02.048.
-
28.
Hosseini A, Rajabian A. Protective effect of Rheum turkestanikum root against doxorubicin-induced toxicity in H9c2 cells. Revista Brasileira de Farmacognosia REV BRAS FARMACOGN. 2016;26(3):347-51. https://doi.org/10.1016/j.bjp.2016.02.004.
-
29.
Yan H, Peng ZG, Wu YL, Jiang Y, Yu Y, Huang Y, et al. Hypoxia-simulating agents and selective stimulation of arsenic trioxide-induced growth arrest and cell differentiation in acute promyelocytic leukemic cells. Haematologica. 2005;90(12):1607-16. [PubMed ID: 16330433].
-
30.
Gupta K, Stefan T, Ignatz-Hoover J, Moreton S, Parizher G, Saunthararajah Y, et al. GSK-3 inhibition sensitizes acute myeloid leukemia cells to 1,25D-mediated differentiation. Cancer Res. 2016;76(9):2743-53. [PubMed ID: 26964622]. [PubMed Central ID: PMC5563074]. https://doi.org/10.1158/0008-5472.CAN-15-2290.
-
31.
Shoeb M. Anticancer agents from medicinal plants. Bangladesh J Pharmacol. 2008;1(2):35-41. https://doi.org/10.3329/bjp.v1i2.486.
-
32.
Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100(1-2):72-9. [PubMed ID: 16009521]. https://doi.org/10.1016/j.jep.2005.05.011.
-
33.
Estan MC, Calvino E, Calvo S, Guillen-Guio B, Boyano-Adanez Mdel C, de Blas E, et al. Apoptotic efficacy of etomoxir in human acute myeloid leukemia cells. Cooperation with arsenic trioxide and glycolytic inhibitors, and regulation by oxidative stress and protein kinase activities. PLoS One. 2014;9(12). e115250. [PubMed ID: 25506699]. [PubMed Central ID: PMC4266683]. https://doi.org/10.1371/journal.pone.0115250.
-
34.
Suganuma K, Miwa H, Imai N, Shikami M, Gotou M, Goto M, et al. Energy metabolism of leukemia cells: Glycolysis versus oxidative phosphorylation. Leuk Lymphoma. 2010;51(11):2112-9. [PubMed ID: 20860495]. https://doi.org/10.3109/10428194.2010.512966.
-
35.
Kuete V, Wabo HK, Eyong KO, Feussi MT, Wiench B, Krusche B, et al. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS One. 2011;6(8). e21762. [PubMed ID: 21886765]. [PubMed Central ID: PMC3158745]. https://doi.org/10.1371/journal.pone.0021762.