Abstract
Objectives:
This study aimed to screen Thais savignyi whole body extracts for antibacterial activity against clinical isolates of some human pathogenic bacteria and analyze its biochemical compounds, including carbohydrate, proteins, alkaloids, cholesterol, unsaturated fatty acids, as well as functional groups and elements.Methods:
Well agar diffusion was used for screening of acetone and methanol extracts against clinical isolates of E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, and Staphylococcus aureus. Biuret and SDS-PAGE methods were applied for quantitative and qualitative protein evaluation. Anthrone method was used for quantitative and qualitative analysis of carbohydrates. Wagner and Mayer's reagents were employed for alkaloid screening. The Liebermann-Burchard test was performed for the detection of cholesterol, infrared for identifying functional groups, and Dumas method for elemental analysis.Results:
Acetone and methanol crude extracts at 0.2 mg/mL concentration inhibited the growth of all the test bacteria; however, the methanol extract was more effective than the acetone extract. The maximum zone of inhibition of the methanol extract was observed against B. cereus (16 mm). Minimal inhibitory concentration of the most effective fraction ranged between 12.5 and 100 µg/mL. Elemental analysis of the bioactive fraction showed 41.55% carbon, 9.49% hydrogen, 6.13% nitrogen, and 1.64% sulfur. IR spectra confirmed the presence of aliphatic, alkenes, secondary amines, and disulfide groups in the most effective fraction of methanol extract of Thais savignyi. Carbohydrate and protein analysis revealed 4.5% carbohydrate and 9% protein. SDS-PAGE disclosed 5 separate bands with 70, 53, 41, 30, and 22 kilodaltons. Wagner and Mayer test showed the presence of alkaloids; mercuric and iodine solutions indicated the presence of unsaturated fatty acids; cholesterol was detected by Liebermann-Burchard reaction and Salkowski test.Conclusions:
The findings of this study suggest that Thais savignyi has antibacterial potential and can be recommended as a source of bioactive compounds of medicinal value.Keywords
Agar Diffusion Antibacterial Activity Bioautography Gastropod Biochemical Analysis Persian Gulf Thais savignyi
1. Background
The rise of antimicrobial resistance toward different conventional antibiotics has been documented. Over the past four decades, scientists around the world have been intrigued with the search for new antimicrobial agents of natural origin (1-4). Various environments including marine resources have been investigated (5). The marine environment comprises a complex ecosystem with a plethora of organisms, many of which are known to possess bioactive compounds as a common means of defense. The marine natural products have been investigated predominantly for their antimicrobial, cytotoxic, antitumor, and anti-inflammatory properties (6). The first attempt to locate antimicrobial activity in marine organisms was initiated around 1950’s (7). Since then, large numbers of marine organisms from a wide range of phyla have been screened for antimicrobial activity (8). Numerous metabolites from marine organisms have been extracted and tested for a variety of ailments. New bioactive compounds from marine sources are estimated to be 10,000 (9).
Gastropods, more commonly known as snails and slugs, are a large taxonomic class within the phylum Mollusca. The class Gastropoda has an extraordinary diversification of habitats. They have the greatest numbers of named mollusc species. However, estimate of the total number of gastropod species varies widely, depending on cited sources (10-12). Some of the more familiar and better-known gastropods are terrestrial gastropods (the land snails and slugs), while some others live in freshwater. However, more than two thirds of all named species live in marine environments. Gastropods have a worldwide distribution from the near Arctic and Antarctic zones to the tropics (11).
The most frequent species in Bushehr province, Iran, are Planaxis sulcatus, followed by Thais savignyi, Turitella fultoni, and Thais mutabilis (10). Thais savignyi is a marine snail from the Gastropods mollusc in the family Muricidae. It has been documented in literature that Gastropods possess potent antimicrobial activity (13). However, bioactivity potential of Thais savigni has not been thoroughly exploited and data on this marine organism are very scanty. Due to the scarcity of data and regarding the current global problem of antimicrobial resistance, and in light of the bioactive potential possessed by marine organisms in the Persian Gulf area, this study was undertaken to screen and characterize methanol and acetone extracts of Thais Savignyi from Bushehr province in the Persian Gulf against some selected human pathogenic bacteria.
2. Methods
2.1. Collection of Samples
The gastropod Thais savignyi from the intertidal zone of the Persian Gulf, Bushehr province, were sampled by hand packing (14) during February and October 2014 and authenticated by Dr. Sayed Mohammad Bagher Nabavi (Khoramshahr Marine Science and Technology University). Freshly collected samples were cleaned and washed with seawater to remove impurities; then they were transferred to the laboratory. Upon arrival to the laboratory, they were rinsed with sterile water and stored at -4°C until use.
2.2. Extraction of Samples
Samples were dried at room temperature. 200-gram portions were extracted separately by addition of 200 mL of acetone and methanol, in sequence. After maceration with continuous shaking for 72 hours, the samples were filtered through Whatman filter paper (No. 1). The solvent was concentrated under reduced pressure to give the respective extracts of acetone (1.56 g) and methanol (1.45 g) (15, 16).
2.3. Test Microorganisms
A total of 5 clinical isolates including E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, and Staphylococcus aureus were obtained on nutrient agar slants from Golestan University hospital in Ahvaz. Initially, all the test bacteria were streaked on nutrient plates for assurance of purity. Then, each bacterial strain was confirmed by biochemical tests (17).
2.4. Bioassay of Crude Extracts
A single isolated colony of an overnight growth at 37 ± 2°C for each bacterial strain was inoculated into brain heart infusion broth and incubated at 37 ± 2°C for 4 - 6 hours until the inoculums turbidity reached 1 ≥ 0.1 OD at 620 nm or 0.5 McFarland standard. Antimicrobial activity of methanol and acetone extracts of Thais savignyi was evaluated by the well agar diffusion method (18). Wells of 7 mm in diameter were made on nutrient agar palates; each plate was seeded with 100 µL pre-adjusted inocula of different bacteria. Using a micropipette, 100 and 200 µL of each 0.2 g/mL crude extract were introduced into each well. The plates were kept inside the refrigerator at 4°C for one hour to allow pre-diffusion of the extract into the medium. All plates were carried out in triplicates and control experiments were also set up by adding different solvents. The plates were incubated at 37°C for 24 hours. The antibacterial activity of the extracts was expressed as the diameter of the inhibition zones (in mm) which appeared on the incubated plates. The plates were kept for one week at room temperature to observe the overgrowth in the zone diameter (19).
2.5. Fractionation
The crude methanol extract exhibited higher effectiveness against all the test bacteria. A portion of the methanol extract (1.5 g) was chromatographed on a silica gel column and eluted with a mixture of n-Hexane/Ethyl acetate with increasing polarity (14), yielding 205 fractions of 10 mL each. The fractions were monitored by TLC and combined according to their similarities. Fractions 1 to 27 were combined, yielding 0.14 mg, while fractions 28 to 43, 44 to 62, 63 to 74, 111 to 129, and 130 to 137 yielded 0.2, 0.3, 0.18, 0.16, and 0.12 mg, respectively.
2.6. Bioautography Screening of Fractions
Antimicrobial activity of the effective fraction was demonstrated and confirmed by bioautography (20). Agar overlay was adopted for evaluation of the active fraction. TLC plates were aseptically placed in sterile Petri plates; pre-seeded nutrient agar was poured over the TLC plates and allowed to solidify, then incubated overnight at 37°C. Following incubation period, the plates were sprayed with MTT (0.8 mg/mL) growth indicator and re-incubated at 37°C for additional 4 hours, then examined for the zone of inhibition.
2.7. Determination of Minimal Inhibitory Concentration (MIC)
MIC of the effective fraction was determined according to the Eloff method (21). A sterile 96-well microtiter-plate was used. A solution containing 50 mg/mL of the effective fraction was prepared. Each well was filled with 100 µL Mueller Hinton Broth (MHB). The test sample (100 µL) was added to the first well, mixed thoroughly and serial two- fold dilutions were made to the desired minimum concentration. Overnight cultures of bacterial suspension (50 µL/well) measuring 0.5 on the McFarland Standard turbidity were added to each well. The plates were incubated at 37°C overnight. A drop of P-nitrotetrazolium chloride solution was added to each well and the plates were incubated for additional 2 hours. The MIC was determined as the lowest sample concentration developing no red color (indicating bacterial growth).
2.8. Biochemical Characterization
The most effective bioactive fraction of Thais savignyi was subjected to qualitative and quantitative analyses. Quantitative estimation of biomolecules was followed using standard methodologies for the estimation of carbohydrates (anthrone reagent method) (22), proteins (Biuret method) (23), and elements (Dumas method) (24).
2.9. Estimation of Total Carbohydrates
For the estimation of carbohydrates (25), 0.5 mL (0.01 mg/mL) of leakage solution was taken and 4 mL of freshly prepared anthrone (150 mg of pure anthrone + 100 mL of sulfuric acid 72%) was added. Then, it was placed in boiling water bath for 10 minutes. Thereafter, the samples were cooled and absorption was read at 625 nm with a spectrophotometer. To establish a standard curve, pure glucose solutions at concentrations of 0.008, 0.01, 0.02, 0.04, 0.1, and 0.2 mg/mL were prepared.
2.10. Estimation of Proteins
Protein molecular size of the bioactive fraction was estimated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (26). In this experiment, resolving gel 12% and staking gel 5% were used. Effective fraction of methanol extract (10 mg) was dissolved in 100 Landa phosphate buffered saline. 10 Landa lysis buffer was added to one-third of its volume, sonicated and centrifuged in a refrigerated centrifuge at 1500 rpm for 30 minutes. The supernatant (40 μL) was mixed with 10 μL of buffer and placed at 97ºC for 5 minutes, then mixed with spin quick for one minute. Then, 50 μL of the sample and 10 μL of 9-235 KDa Lader were run over gel and an electrical current of 130 volts was established for 2 hours. At the end of the experiment, the gel was exposed with comasis blue.
2.11. Elemental Analysis
Sample was burned with pure oxygen; gases from the burning materials automatically were measured by means of a device. Carbon, hydrogen, nitrogen, and sulfur elements in the sample were detected (24).
Qualitative analysis of the most bioactive fraction of Thais savignyi included: functional groups determination (FTIR analysis); identification of alkaloids (Wagner and Mayer’s test), cholesterol determination (Liebermann-Burchard reaction and Salkowski test), and unsaturated fatty acids (chloromercurric method).
2.12. Functional Group Determination
To determine the functional groups of most bioactive fraction extract, a portion of the sample was freeze-dried, ground to powder, mixed with potassium bromide, and compressed as a disc. The disc was placed inside infrared spectrometer and the results were recorded.
3. Results
3.1. Antibacterial Assay
Antibacterial potential of acetone and methanol extracts of Thais savignyi was evaluated against 5 clinical isolates by the well agar diffusion method. The results are summarized in Table 1. As shown in this the the highest zone of inhibition was shown by 200 mg/mL methanol extract against B. cereus (16 mm).
Diameters of Inhibition Zone (mm) Exhibited by Methanol and Acetone Thais Savigni Extract Against the Test Bacteria
| Solvent | Amount of Extract, μL | Bacillus cereus | Escherichia coli | Staphylococcus aureus | Klebsiella pneumoniae | Pseudomonas aeruginosa |
|---|---|---|---|---|---|---|
| Methanol | 100 | 12 | 6 | 8 | 5 | 6 |
| 200 | 16 | 8 | 11 | 9 | 9 | |
| Acetone | 100 | 10 | 5 | 6 | 5 | 6 |
| 200 | 15 | 6 | 6 | 6 | 7 |
The methanol extract was fractionated by column chromatography and the bioactivity potential was confirmed by bioautography.
The MIC of the most effective fraction was determined to be 25 µg/mL, 25 µg/mL, 50 µg/mL, 100 µg/mL, and 1.25 µg/mL for E. coli, S. aureus, K. pneumoniae, P. aeruginosa, and B. cereus, respectively.
Biochemical Characterization
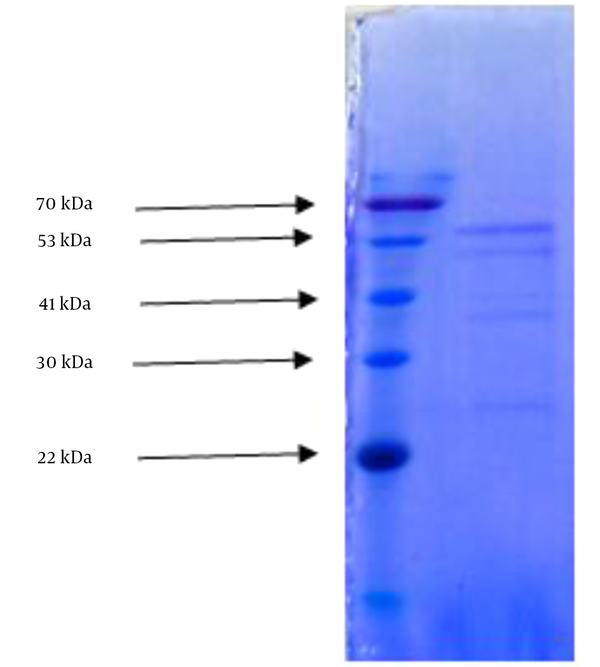
Carbohydrate and protein detection yielded 4.5% and 9% respectively in the most effective fraction of methanol extract of Thais savigni. SDS-AGE disclosed 5 separate bands with 70, 53, 41, 30, and 22 kilo Dalton (Figure 1).
SDS-PAGE Determination of Purified Proteins Molecular Size
Wagner and Mayer test showed the presence of alkaloids; mercuric and iodine solutions indicated the presence of fatty acids; Liebermann-Burchard and Salkowski tests revealed the existence of cholesterol.
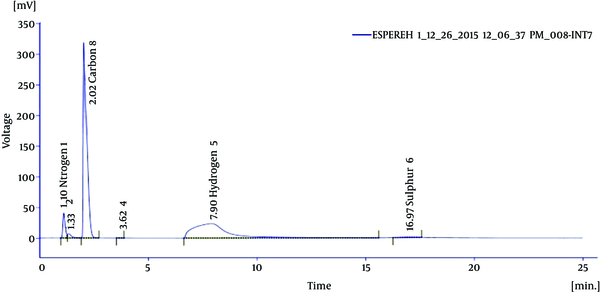
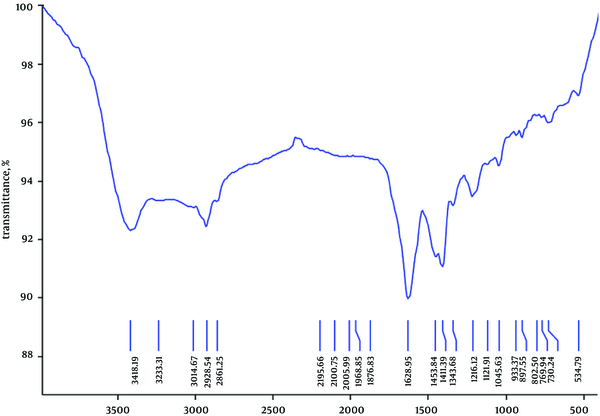
Elemental analysis of the effective fraction revealed 41.55% carbon, 9.49% hydrogen, 6.13% nitrogen, and 1.64% sulfur (Figure 2). The functional group of the IR spectrum revealed that the fraction showing the most antibacterial activity may be of aliphatic (2861 and 3014 cm-1), alkenes (1628 cm-1), secondary amines (1045 and 3415 cm-1), and disulfide (534 cm-1), as presented in Figure 3.
Elemental Analysis of Most Effective Fraction of Thais Savigni Methanol Extract
FTIR Spectrum of Most Effective Fraction of Thais Savigni Methanol Extract
4. Discussion
As the emergence of antimicrobial resistance and the onset of adverse effects of synthetic antibiotics increase, the search for antimicrobial agents of natural sources continues (27). Over the past few decades, efforts around the world have been devoted to isolate and screen various marine organisms for various bioactive potentials toward different ailments including antimicrobial activity (28).
Marine organisms possess a wide range of bioactive compounds as a means of defense mechanisms. Many marine molluscs have evolved chemical defense mechanisms for their eggs and thus produce secondary metabolites which possess antimicrobial activities (29). They are considered as important natural sources to derive many valuable compounds that exhibit various bioactivities including antimicrobial effectiveness (30).
Thais savignyi is a Gastropod that has been located and identified in the seaside of Bushehr; however, despite its abundance in the Persian Gulf (18), its bioactivity has not been thoroughly explored. In this investigation, Thais savignyi was screened for antimicrobial activity against 5 species of common human pathogenic bacteria, using methanol and acetone as bioactive compounds extractor solvents.
Overall, our results showed that both methanol and acetone extracts at applied concentrations (100 and 200 µL) inhibited the growth of all the 5 test bacteria. However, the highest effect was observed by the methanol extract (200 µL) against Bacillus cereus; thus it was selected for more detailed study. In addition, the results obtained from the current study were compared with different conventional commercially used antibiotics that showed that gentamycin inhibited the growth of E. coli, B. cereus, and P. aeruginosa with zone inhibitions of 9, 10, and 12 mm, respectively. Ciprofloxacin showed a significant effectiveness (15 mm inhibition zone) against E. coli only.
Although a high number of investigations have been performed on a vast variety of marine life, data on the bioactivity of Thais savignyi are unavailable in literature. However, similar studies have been conducted on different marine organisms including Gastropods (12, 31-33).
Ramasamy et al. (33) screened antibacterial drugs from different solvent extracts of tissue and egg of the marine gastropods Chicoreus ramosus against different clinical isolates of human pathogenic bacteria for their activity. They reported that the acetone and chloroform of the tissue and egg inhibited the growth of the test bacteria.
Similarly, Sugesh et al. (12) studied the antibacterial activity of ethanol, methanol, and aqueous extracts of the marine Gastropod Hemifusus Pugilinus against 10 human pathogenic bacteria. In their investigation, they observed the highest activity for ethanol extract toward all the test bacteria followed by methanol and aqueous extracts.
Diane (31) performed a similar study on the antimicrobial potential of gastropods against human pathogenic bacteria employing solvents with different polarities and reported results confirming the findings of other investigators.
In their study, Ramasamy et al. (33) reported the maximum zone of inhibition from acetone extract against S. paratyphi A, which is a Gram-negative bacterium.
On the contrary, in our study, we observed weak to moderate activity by the acetone extract against all the test bacteria used in the study. Nonetheless, more pronounced activity was exhibited by the methanol extract against B. cereus which is a Gram-positive bacterium with MICs ranging between 25 µg/mL and 1.25 µg/mL.
Although various species of Gastropods have been investigated for their antimicrobial functionalities by different experimental methods and different solvents, they revealed similar results with varying degrees of antimicrobial potential.
In our study, the higher activity of methanol extract may be due to the greater solubility of the extract in this solvent. In other words, it can be stated that methanol is a suitable solvent for the bioactive compounds present in Thais savignyi. These results encourage the idea that marine Gastropods are potent sources for antibacterial drug development.
Even though data on the bioactivity of Thais savignyi are very limited, this marine animal, due to its availability (10) and medicinal utility, can be considered as candidate for studying novel marine compounds for drug discovery in the Persian Gulf.
The present study indicated that the Thais savignyi methanol extract contains compounds with antibacterial activity. However, further investigations involving isolation, purification, and identification of the active compounds and possible mode of action as drugs for humans are needed. In this regard, preliminary step done in the present study was fractionation of crude bioactive extract, by column chromatography. Bioautography showed that the 3rd and 4th fractions from 6 fractions prepared and identified by TLC from methanol extract have more impact on the bacteria.
Peptides, nitrogenous compounds, monosaccharides, fatty acids, polypeptides, and sterols have been identified and characterized in antibacterial extracts of marine gastropods in similar studies (34).
Carbohydrates are the human body key sources of energy (35). It was found that the amount of carbohydrate in the sample was 4.5%.
Proteins act as enzymes, hormones, and antibodies and are responsible for the formation of bones, teeth, hair, and the outer layer of skin; they also help maintain the structure of blood vessels and other tissues (36). Based on our results, the amount of protein in the sample was 9%. SDS-PAGE showed 5 bands weighing 71, 53, 41, 30, and 22 kDa, which denotes five types of proteins.
Alkaloids are naturally occurring chemical compounds containing basic nitrogen atoms. They often have pharmacological effects and are used as medications and recreational drugs. Alkaloids, unsaturated fatty acids, and cholesterol identified in the effective fractions of methanol extract of Thais savigni could be responsible for its antibacterial activity (37).
In biological molecules, functional groups play an important role in the formation of such molecules as proteins and carbohydrates. Aldehydes and ketones are active groups in carbohydrates. Monosaccharides contain free aldehyde or ketone groups. Some disaccharides have free aldehyde groups (maltose) and some do not have the free ones (sucrose). Infrared spectrum of the most effective fraction of methanol extract showed the presence of aliphatic, alkenes, secondary amines, and disulfide. Carbohydrates were composed of only three atoms: carbon, hydrogen, and oxygen, as confirmed by elemental analysis in the present study.
4.1. Conclusion
Data obtained from the present study indicated that the acetone and methanol extracts of Thais savignyi inhibited the growth of Gram positive and Gram negative bacteria in vitro. Moreover, the present study conclusively demonstrates that Thais savignyi is a good source of various biochemical compounds like alkaloids, proteins, carbohydrates, Cholesterol, and fatty acids. Thus, this gastropod is considered to be of value for future study to find medicinal drugs as a sustainable natural source.
References
-
1.
Mansour A, Sepideh S, Mohammad H. Antimycobacterial activity of partial purified extract of Allium ascalonicum. Jundishapur J Microbiol. 2009;2009(4, Autumn):144-7.
-
2.
Adibpour N, Nasr F, Nematpour F, Shakouri A, Ameri A. Antibacterial and Antifungal Activity of Holothuria leucospilota Isolated From Persian Gulf and Oman Sea. Jundishapur J Microbiol. 2014;7(1). e8708. [PubMed ID: 25147657]. https://doi.org/10.5812/jjm.8708.
-
3.
Amin M, Rakhisi Z, Zarei Ahmady A. Isolation and identification of bacillus species from soil and evaluation of their antibacterial properties. Avicenna J Clin Microb Infec. 2015;2(1). https://doi.org/10.17795/ajcmi-23233.
-
4.
Yousefimanesh H, Amin M, Robati M, Goodarzi H, Otoufi M. Comparison of the Antibacterial Properties of Three Mouthwashes Containing Chlorhexidine Against Oral Microbial Plaques: An in vitro Study. Jundishapur J Microbiol. 2015;8(2). e17341. [PubMed ID: 25825646]. https://doi.org/10.5812/jjm.17341.
-
5.
Evans CE, Banso A, Samuel OA. Efficacy of some nupe medicinal plants against Salmonella typhi: an in vitro study. J Ethnopharmacol. 2002;80(1):21-4. [PubMed ID: 11891083]. https://doi.org/10.1016/S0378-8741(01)00378-6.
-
6.
Anand TP, Rajaganapathi J, Edward JK. Antibacterial activity of marine molluscs from Portonovo region. Indian J Mar Sci. 1997;26:206-8.
-
7.
Burkholder PR, Burkholder LM. Antimicrobial activity of horny corals. Science. 1958;127(3307):1174-5. [PubMed ID: 13555859]. https://doi.org/10.1126/science.127.3307.1174.
-
8.
Shaw PD, McClure WO, Van Blaricom G, Sims J, Fenical W, Rude J. Antimicrobial activities from marine organisms. Fooddrugs from the sea. Washington DC: Marine Technology Society; 1976. p. 55-60.
-
9.
Kelecom A. Secondary metabolites from marine microorganisms. An Acad Bras Cienc. 2002;74(1):151-70. https://doi.org/10.1590/s0001-37652002000100012.
-
10.
Kohan A, Badbardast Z, Shokri M. The Gastropod fauna along the Bushehr province intertidal zone of the Persian Gulf. J Persian Gulf. 2012;3(9):33-42.
-
11.
Lindberg DR, Ponder WF, Haszprunar G. The Mollusca: relationships and patterns from their first half-billion years. Assembling the tree of life. Oxford University Press; 2004. p. 252-78.
-
12.
Sugesh S, Mayavu P, Ezhilarasan P, Sivashankar P, Arivuselvan N. Screening of antibacterial activities of marine gastropod hemifusus pugilinus. Curr Res J Biol Sci. 2012;5(2):49-52.
-
13.
Anand TP, Edward JK. Antimicrobial activity in the tissue extracts of five species of cowries Cypraea spp.(Mollusca: Gastropoda) and an ascidian Didemnum psammathodes (Tunicata: Didemnidae). Indian J Marine Sci. 2002;31(3):239-42.
-
14.
Chellaram C, Edward JKP. In vivo anti-inflammatory bustle of reef associated mollusc, Trochus tentorium. Adv Biotech. 2009;8(12):32-4.
-
15.
Sarumathi G, Arumugam M, Kumaresan S, Balasubramanian T. Studies on bioprospecting potential of a gastropod mollusc Cantharus tranquebaricus (Gmelin, 1791). Asian Pac J Trop Biomed. 2012;2(10):759-64. [PubMed ID: 23569843]. https://doi.org/10.1016/S2221-1691(12)60225-1.
-
16.
Vimala P, Thilaga RD. Antibacterial activity of the crude extract of a gastropod lambis lambis from the Tuticorin Coast, India, W. App Sci J. 2012;16(10):1334-7.
-
17.
Tille P. Bailey & Scott's diagnostic microbiology-E-book. Elsevier Health Sciences; 2013.
-
18.
Stoke JE. Clinical Bacteriology. London: Edward Arnold Ltd; 1980.
-
19.
Ramasamy P, Thampi DPK, Chelladurai G, Gautham N, Mohanraj S, Mohanraj J. Screening of antibacterial drugs from marine gastropod Chicoreus ramosus. J Coast Life Med. 2013;1(3):181-5.
-
20.
Dewanjee S, Gangopadhyay M, Bhattacharya N, Khanra R, Dua TK. Bioautography and its scope in the field of natural product chemistry. J Pharm Anal. 2015;5(2):75-84. https://doi.org/10.1016/j.jpha.2014.06.002.
-
21.
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64(8):711-3. [PubMed ID: 9933989]. https://doi.org/10.1055/s-2006-957563.
-
22.
Hansen J, Moller I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem. 1975;68(1):87-94. [PubMed ID: 1190454].
-
23.
Peterson GL. Determination of total protein. Enzymol. 1983;91:95-119. https://doi.org/10.1016/s0076-6879(83)91014-5.
-
24.
Gel'man NE, Terent'eva EA, Shanina TM, Kiparenko LM, Rezl V. Methods of quantitative organic elemental microanalysis. Khimiya. 1987:226.
-
25.
Olennikov DN, Tankhaeva LM. Absorption spectra of carbohydrates and related compounds in H2SO4. Chem Natural Compounds. 2006;42(3):262-4. https://doi.org/10.1007/s10600-006-0095-5.
-
26.
Periyasamy N, Srinivasan M, Balakrishnan S. Antimicrobial activities of the tissue extracts of Babylonia spirata Linnaeus, 1758 (Mollusca: Gastropoda) from Thazhanguda, southeast coast of India. Asian Pac J Trop Biomed. 2012;2(1):36-40. [PubMed ID: 23569831]. https://doi.org/10.1016/S2221-1691(11)60186-X.
-
27.
Fernandes P. Antibacterial discovery and development--the failure of success? Nat Biotechnol. 2006;24(12):1497-503. [PubMed ID: 17160049]. https://doi.org/10.1038/nbt1206-1497.
-
28.
Debbab A, Aly AH, Lin WH, Proksch P. Bioactive compounds from marine bacteria and fungi. Microb Biotechnol. 2010;3(5):544-63. [PubMed ID: 21255352]. https://doi.org/10.1111/j.1751-7915.2010.00179.x.
-
29.
Benkendorff K, Davis AR, Bremner JB. Chemical defense in the egg masses of benthic invertebrates: an assessment of antibacterial activity in 39 mollusks and 4 polychaetes. J Invertebr Pathol. 2001;78(2):109-18. [PubMed ID: 11812113]. https://doi.org/10.1006/jipa.2001.5047.
-
30.
Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo). 2005;58(1):1-26. [PubMed ID: 15813176]. https://doi.org/10.1038/ja.2005.1.
-
31.
Defer D, Bourgougnon N, Fleury Y. Screening for antibacterial and antiviral activities in three bivalve and two gastropod marine molluscs. Aquaculture. 2009;293(1-2):1-7. https://doi.org/10.1016/j.aquaculture.2009.03.047.
-
32.
Suresh M, Arularasan S, Srikumaran N. Screening on antimicrobial activity of marine gastropods Babylonia zeylanica (Bruguière, 1789) and Harpa conoidalis (Lamarck, 1822) from Mudasalodai, southeast coast of India. Int J Pharm Pharm Sci. 2012;4(4):552-6.
-
33.
Ramasamy P, Padmakumari D, Thampi K, Chelladurai G, Gautham N, Mohanraj S. Screening of antibacterial drugs from marine gastropod Chicoreus ramosus (Linnaeus, 1758). J Coast Life Med. 2013;1(3):181-5.
-
34.
Alam M,, Thomson RH. Handbook of natural products from marine invertebrates. CRC Press; 1998.
-
35.
Pamela C, Richard A, Denise R. Lippincotts illustrated Reviews Biochemistry. Philadelphia: Lippincott Williams and Wilkins; 2005.
-
36.
Jayaseelan M, Arumugam T, Kumar PS, Thangaraj N. Biochemical Quantification and antibacterial properties of Corallocarpus epigaeus. Biosci discov. 2016;7(1):11-6.
-
37.
Rhoades DF. Evolution of plant chemical defense against herbivores. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press; 1979. p. 3-54.