Abstract
Background:
Neonatal hyperbilirubinemia, a high-incidence disease, arises from an imbalance of bilirubin production and elimination. Neonatal jaundice requires medical attention in the clinic. Finding new therapeutic approaches, besides available strategies such as phototherapy, seems necessary. In traditional beliefs Cichorium intybus could exert hepatoprotective effects.Objectives:
In this study we evaluated the effect of administration of hydroalcoholic Cichorium intybus extract to the lactating rats in δ-aminolaevulic acid-induced hyperbilirubinemia in rat neonates.Methods:
We injected δ-aminolaevulic acid (i.p, 80 micromolar) three times at the intervals of 4 hours to rat newborns (7 to 10-day-old). Cichorium intybus extract (p.o, 25 and 50 mg/kg) was administered to lactating rats at the time of interval of 2 hours (0 - 10 hours). After neonatal feeding, we assessed the bilirubin plasma levels in 0, 8, 16, and 48 hours.Results:
Injection of δ-aminolaevulic acid significantly enhanced the conjugated and total bilirubin concentration in rat newborns. Maternal administration of Cichorium intybus extract at the dose of 25 and 50 mg/kg led to significant reduction of bilirubin plasma levels in neonates after feeding. The dose of 50 mg/kg showed a more potent effect.Conclusions:
Taken together, treatment of lactating rats with Cichorium intybus extract exerts protective effects in the δ-aminolaevulic acid-induced hyperbilirubinemia of rat neonates.Keywords
1. Background
Neonatal hyperbilirubinemia, icterus neonatorum, is a very common phenomenon in the clinic (1). This condition may have harmful consequences such as acute encephalopathy and bilirubin-induced neurological dysfunction and kernicterus (2). Pathologic jaundice is a result of excessive production of bilirubin, exaggeration in erythrocyte destruction, hepatic uptake deficiency, impaired conjugation of bilirubin, enhanced bilirubin enterohepatic circulation, and increased rate of tissue heme oxidation (3, 4).
Bilirubin, a bile pigment, is synthesized in a metabolic pathway from catabolism of hemoglobin of senescent or hemolyzed red blood cells in mammals (3, 5). Bilirubin could be considered as an antioxidant agent at low concentrations, which defines its physiologic benefits, however, its deposition during hyperbilirubinemia exerts cellular injury (3, 6-8). Bilirubin interferes with synthesis of DNA or proteins, induces DNA strand breakage, prevents mitochondrial function, and impairs the function of N-methyl-D-aspartate receptors. Bilirubin can also affect nerve conduction (notably auditory nerve) and inhibit renal function (9-12). Based on the cellular toxic effects of bilirubin and pathophysiology of neonatal hyperbilirubinemia, development of new strategies for prevention, detection, and treatment of this disease are of great importance.
Cichorium intybus L., a member of the Compositae family, with the common name of chicory, has long been used in folklore medicine (13). Chicory possesses many beneficial properties in treatment of gastroenteritis, gallstones, acne, inflammation, splenomegaly, sinus problems, diarrhea, and vomiting. Administration of leaves, roots, seeds, and fruit extract of this medical plant could be anti-diabetic, diuretic, antioxidant, and immunomodulator (14-18).
Several studies reported that Cichorium intybus has potent hepatoprotective effects and has been used as a traditional therapy against jaundice. Saggu et al., 2014, showed that Cichorium intybus fruit extract could ameliorate the oxidative stress and hepatic damage induced by 4-tert-OP compound. Jamshidzadeh et al., 2006, showed that hepatoprotective ability of chicory leaves extract on carbon tetrachloride induced toxicity (19).
As there are not enough experimental studies on the role of Cichorium intybus in neonatal hyperbilirubinemia, in the current investigation we examined the modulatory effect of chicory extract on the rat infant hyperbilirubinemia, which was induced via the standard model of delta-aminolevulinic acid. In this experiment the plant extract was administered to lactating mothers in order to study its protective properties in neonates with the hope of identification of new therapeutic approaches.
2. Methods
2.1. Experimental Animals
This study was performed on adult female wistar rats weighing 200 - 220 g and their neonates with the age of 7 - 10 days (Rafsanjan University of Medical Sciences, Iran). They were housed in the standard cages under controlled laboratory environment (temperature: 24 ± 1°C, humidity: 55 ± 10%, lighting: 12-hours light/dark cycle) with standard laboratory pellet chow and water. All the procedures were conducted according to the institutional guideline for the care and use of laboratory animals with the approval of Rafsanjan University research and Medical ethics committees. Each animal was used only once and each group consisted of 6 animals.
2.2. Preparation of the Hydroalcoholic Extract
Aerial parts of Cichorium intybus were air-dried under shade and grinded. These samples were botanically identified at botany research division, research institute of forests and rangelands, Tehran, Iran. Dried powder (200 g) was extracted by the percolation method using a hydroalcohol solution (ethanol/water 80% v/v). The resultant mixture was filtered and concentrated in reduced pressure (at 30 - 40°C). The hydroalcoholic extract was prepared in sterile isotonic saline solution at 25 and 50 mg/kg in order to evaluate its bilirubin reduction property.
2.3. Hyperbilirubinemia Induction
Experimental postnatal hyperbilirubinemia was induced by administration of delta-aminolevulinic acid (ALA), the heme precursor, between 7 - 15 postnatal days. Injection of ALA in this time period resulted in rapid and consistent elevation in bilirubin serum level in developing rat neonates. We utilized this model of experimental jaundice to examine the probable ability of Cichorium intybus extract in suppressing the hyperbilirubinemia (20). This agent was purchased from Sigma-Aldrich Corporation.
2.4. Experimental Design
We injected ALA at the dose of 80 micromolar (i.p) to rat neonates in the time of interval of 0, 4, and 8 hours to induce the hyperbilirubinemia. Plasma bilirubin was measured at 0, 8, 16, 24, and 32 hours after the last injection of ALA (based on the method of Jendrassik and Grof). Rat mothers continued lactation to their neonates until bilirubin measurement time. To clarify the effect of plant extract on neonatal jaundice, we designed 4 experimental groups as follows: 1, control, that didn’t receive ALA (to neonates) + normal diet (to mothers); 2, ALA, the group that received ALA (to neonates) + normal diet (to mothers); 3, ALA + 25 mg/kg, the groups that received ALA (to neonates) + 25 mg/kg chicory (to mothers), and 4, ALA + 50 mg/kg, the groups that received ALA (to neonates) + 50 mg/kg chicory (to mothers). Our extract was administered orally in 0, 2, 4, 6, 8, and 10 hours. Each group was divided to five sub-groups (N = 6 per sub-group) based on different times (0, 4, 8, 16, 24, 32) of bilirubin measurement.
2.5. Bilirubin Assay
For this measurement, blood samples were obtained via direct heart puncture during ketamine-induced anesthesia, from vehicle and Cichorium intybus extract-treated animals. The samples were centrifuged at 1000 × g for 10 minutes to separate the plasma and then stored at -20°C until the assay. Quantitative detection of bilirubin plasma level was performed with a calorimetric diazo method (21). Plasma bilirubin concentrations are expressed as mg/dL.
2.6. Statistics
Data were presented as mean ± standard error of the mean (S.E.M). The computations were done by SPSS software package (version 16), Excel (Microsoft Excel, 2010, Microsoft Corporation, USA), and Graph-pad prism software version 6. Results were analyzed by one-way analysis of variance (ANOVA) with tukey post hoc test. P value < 0.05 was defined significant for all analyses.
3. Results
3.1. Attenuation of Total Plasma Bilirubin in Neonates by Maternal Administration of Cichorium Intybus Extract After ALA-Induced Hyperbilirubinemia
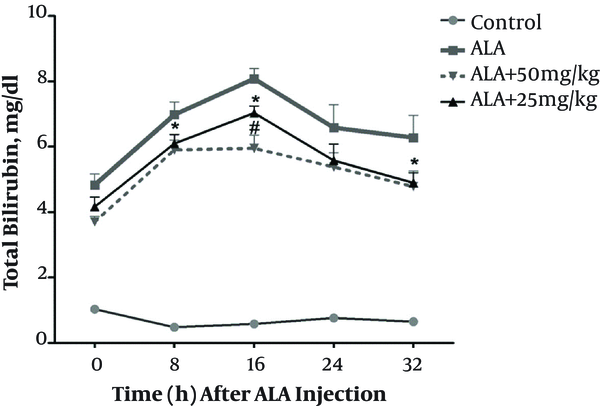
To investigate the effect of chicory on neonatal hyperbilirubinemia, ALA was injected in order to induce this condition. As illustrated in Figure 1, ALA could significantly enhance the neonatal bilirubin level in comparison with control animals and reached to the peak level (8 ± 0.31 mg/dL) on the 16th hour after injection (all P < 0.001). Neonatal lactation following gavage of chicory extract to mothers (25 mg/kg) led to significant reduction of total bilirubin concentration compared to ALA-treated group on the 8th, 16th, and 32nd hours (all P < 0.05). Similarly, maternal gavage of 50 mg/kg chicory decreased the neonatal total bilirubin level compared to ALA-treated animals on the 8th, 16th, and 32nd hours (all P < 0.05). Comparison between two doses of chicory revealed that 50 mg/kg of chicory is more effective on total bilirubin as the concentration of bilirubin was lower than the groups, which were administered 25 mg/kg chicory (significant in the 16th hours after ALA administration, P = 0.012).
The effect of treatment of mothers with different doses of Cichorium intybus (chicory) on neonatal total bilirubin after delta-aminolevulinic acid (ALA) induced hyperbilirubinemia. Cichorium intybus extract was administered by gavage to rat mothers and neonates were fed by their mothers. The * means significant difference between ALA treated group with the ALA + 25 mg/kg and ALA + 50 mg/kg chicory treated groups in 8, 16, and 32 hours after ALA injection (all P < 0.05). The # means significant difference between ALA + 25 mg/kg with ALA + 50 mg/kg chicory treated groups in 16 hours after ALA injection (P = 0.012). Data are expressed as mean ± S.E.M. control: the group that didn’t receive ALA (to neonates) + normal diet (to mothers), ALA: the group that received ALA (to neonates) + normal diet (to mothers), ALA + 25 mg/kg: the groups that received ALA (to neonates) + 25 mg/kg chicory (to mothers), ALA + 50: the groups that received ALA (to neonates) + 50 mg/kg chicory (to mothers). Horizontal axis shows different times after injection of ALA. For more details please see the methods.
3.2. Effects of Maternal Cichorium Intybus Treatment on the Conjugated Bilirubin Level of Rat Neonates
As illustrated in Figure 2, ALA could increase the neonatal conjugated bilirubin concentrations compared to control and the peak level was observed on the 24th hours after injection (4.2 ± 0.16 mg/dL, all P < 0.001). One way ANOVA revealed a significant conjugated bilirubin diminishing effect for the doses of 25 and 50 mg/kg of chicory (significant at 24 and 32 hours, all P < 0.05). Moreover, our data showed that the dose of 50 mg/kg of chicory has a stronger effect than the dose of 25 mg/kg in reducing the conjugated bilirubin level (significant on the 0 hours, P = 0.001).
The effect of maternal treatment with different doses of Cichorium intybus (chicory) on neonatal conjugated after delta-aminolevulinic acid (ALA)- induced hyperbilirubinemia. The * means significant difference between ALA treated group with the ALA + 25 mg/kg and ALA + 50 mg/kg chicory treated groups in 24 and 32 hours after ALA injection (all P < 0.05). The * means significant difference between ALA + 25 mg/kg with ALA + 50 mg/kg chicory treated groups in 0 hours after ALA injection (P = 0.001). Other notations are similar to Figure 1.

4. Discussion
In the current investigation we demonstrated that repetitive injection of ALA could induce hyperbilirubinemia in rat neonates. Administration of different doses of Cichorium intybus (chicory) extract (25 and 50 mg/kg) to rat mothers resulted in reduction of both total and conjugated plasma bilirubin levels in newborns. The dose of 50 mg/kg of chicory was more effective than the dose 25 mg/kg in this phenomenon.
Cichorium intybus (Compositae/Asteraceae) has long been used as a medical plant in Eurasia and Africa by various folkloric groups (22). In the aspect of phytochemistry, the plant contains chicoric acid, aliphatic compounds, saccharides, flavonoids, essential oils, methoxycoumarin cichorine, anthocyanins, volatile compounds, and is rich in proteins, carbohydrates (inulin), and minerals (23, 24). Cichorium intybus possesses a vast range of pharmacological activities and has been used in plant-based remedies for centuries in different countries, especially Iran.
The therapeutic application of chicory in traditional medicine is related to diabetes, wound healing, tumors, inflammation, digestive disorders, gallstones, gastroenteritis, liver diseases, and jaundice. The extract can also be effective as antipyretic, choleretic, and depurative; its syrup is tonic and purifying for infants (14, 24-28).
Literature review indicated that this medical plant is beneficial in liver problems similar to many other herbal medicines, which exert hepatoprotection (29-32). Zafar and Mujahid Ali reported the anti-hepatotoxic effects of root and root callus extracts of chicory in carbon tetrachloride-induced hepatic damage of rats (33). Ahmed et al., showed that the seeds of Cichorium intybus play a hepatoprotective role (27). Hassan et al., demonstrated that chicory exerts a modulatory role against hepatotoxicity induced by nitrosamine precursors (34).
The main issue for administration of chicory in a clinic is the safety (high therapeutic index). The potential toxicological property is related to high concentration of secondary metabolites (24). The sesquiterpene-rich extract was examined for potential mutagenicity by the Ames test in Salmonella typhimurium and Escherichia coli strains. Despite some cytotoxic effects at high doses, mutagenicity was not observed following chicory treatment (24, 35).
Data from a sub-chronic (28-day) oral toxicity study in rats showed that there are no signs of toxicological effects or mortality related to extract. Another study based on Vibrio ficheri bioluminescence inhibition assay showed that the extract is safe for use in human (24, 35, 36). The main concern regarding the Asteraceae family is the risk of allergic reactions, however, more comprehensive studies are necessary to determine the safety of chicory. In this study chicory, at doses 25 and 50 mg/kg, did not induce any adverse reactions (pilot study). Moreover, in previous studies higher doses (250 or 300 mg/kg) were administered and in other species, for instance Cichorium glandulosum, the administered dose was up to 800 mg/kg in rat (37).
There are limited clinical trials on chicory beneficial usages. One study on patients suffering from osteoarthritis revealed that treatment with chicory is well-tolerated and only one patient was withdrawn from the study due to adverse reaction (in order to highest dose of extract) (38). Another clinical study was designed to assess the intake of chicory coffee as well as cardiovascular protection and showed a new insight for investigating the anti-inflammatory, antithrombotic, and beneficial hemorheologic properties of chicory coffee phenolic compounds (39).
Bilirubin metabolism in the fetus is placenta-dependent and most of the bilirubin remains in the unconjugated form, which can clear through placenta readily/efficiently and then conjugated and excreted by maternal hepatic system. After birth, placenta bilirubin metabolism is replaced by neonatal hepatic system. Any immaturity in hepatic and intestinal processes of conjugation and metabolism leads to physiological jaundice and enhances the risk of neonatal hyperbilirubinemia (40-42).
Two types of jaundice, such as breastfeeding jaundice and breast milk jaundice, are usually harmless and more severe forms are associated with several conditions including metabolic/endocrine disorders, hemolytic diseases, liver anatomic abnormalities, infections (hepatitis, sepsis), and in rare conditions, bilirubin passage across the blood brain barrier causes kernicterus. Bilirubin at low concentration is a potent intracellular antioxidant, which prevents membrane peroxidation and is the scavenger of reactive oxygen species but at higher doses exerts toxic effects (43).
Since neonatal hyperbilirubinemia is a frequently encountered condition in term and preterm infants, designing an efficacious, novel, and safe treatment is essential. In our study, injection of ALA to rat newborns increased bilirubin significantly compared to control. Then, neonates were fed with chicory-rich milk by their mothers that resulted in bilirubin reduction. Our findings are in line with previous studies that demonstrated protective effects of chicory.
In summary, maternal administration of Cichorium intybus extract reduced conjugated and total bilirubin levels in rat neonates following lactation. More studies are needed to clarify the possible mechanism of action as well as the safety profile of this valuable extract to use in lactating mothers.
Acknowledgements
References
-
1.
Rubaltelli FF. Current drug treatment options in neonatal hyperbilirubinaemia and the prevention of kernicterus. Drugs. 1998;56(1):23-30. [PubMed ID: 9664196]. https://doi.org/10.2165/00003495-199856010-00003.
-
2.
Allen NM, Mohammad F, Foran A, Corcoran D, Clarke T. Severe hyperbilirubinaemia and kernicterus: more caution is needed in newborn jaundice surveillance. Ir Med J. 2009;102(7):228-9. [PubMed ID: 19772008].
-
3.
Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med. 2001;344(8):581-90. [PubMed ID: 11207355]. https://doi.org/10.1056/NEJM200102223440807.
-
4.
Shapiro SM. Bilirubin toxicity in the developing nervous system. Pediatr Neurol. 2003;29(5):410-21. [PubMed ID: 14684236]. https://doi.org/10.1016/j.pediatrneurol.2003.09.011.
-
5.
Yueh MF, Chen S, Nguyen N, Tukey RH. Developmental onset of bilirubin-induced neurotoxicity involves Toll-like receptor 2-dependent signaling in humanized UDP-glucuronosyltransferase1 mice. J Biol Chem. 2014;289(8):4699-709. [PubMed ID: 24403077]. [PubMed Central ID: PMC3931032]. https://doi.org/10.1074/jbc.M113.518613.
-
6.
Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987;84(16):5918-22. [PubMed ID: 3475708]. [PubMed Central ID: PMC298974]. https://doi.org/10.1073/pnas.84.16.5918.
-
7.
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043-6. [PubMed ID: 3029864]. https://doi.org/10.1126/science.3029864.
-
8.
Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113(6):1776-82. [PubMed ID: 15173506]. https://doi.org/10.1542/peds.113.6.1776.
-
9.
Bratlid D. How bilirubin gets into the brain. Clin Perinatol. 1990;17(2):449-65. [PubMed ID: 2196140].
-
10.
Sellinger M, Haag K, Burckhardt G, Gerok W, Knauf H. Sulfated bile acids inhibit Na(+)-H+ antiport in human kidney brush-border membrane vesicles. Am J Physiol. 1990;258(4 Pt 2):F986-91. [PubMed ID: 2158747]. https://doi.org/10.1152/ajprenal.1990.258.4.F986.
-
11.
Chuniaud L, Dessante M, Chantoux F, Blondeau JP, Francon J, Trivin F. Cytotoxicity of bilirubin for human fibroblasts and rat astrocytes in culture. Effect of the ratio of bilirubin to serum albumin. Clin Chim Acta. 1996;256(2):103-14. [PubMed ID: 9027422]. https://doi.org/10.1016/S0009-8981(96)06407-8.
-
12.
Hoffman DJ, Zanelli SA, Kubin J, Mishra OP, Delivoria-Papadopoulos M. The in vivo effect of bilirubin on the N-methyl-D-aspartate receptor/ion channel complex in the brains of newborn piglets. Pediatr Res. 1996;40(6):804-8. [PubMed ID: 8947954]. https://doi.org/10.1203/00006450-199612000-00005.
-
13.
Muthusamy VS, Anand S, Sangeetha KN, Sujatha S, Arun B, Lakshmi BS. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem Biol Interact. 2008;174(1):69-78. [PubMed ID: 18534569]. https://doi.org/10.1016/j.cbi.2008.04.016.
-
14.
Pushparaj PN, Low HK, Manikandan J, Tan BK, Tan CH. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111(2):430-4. [PubMed ID: 17197141]. https://doi.org/10.1016/j.jep.2006.11.028.
-
15.
Ahmed N. Alloxan diabetes-induced oxidative stress and impairment of oxidative defense system in rat brain: neuroprotective effects of Cichorium intybus. Int J Diabetes Metab. 2009;17:105-9.
-
16.
Hassan H. The prophylactic role of some edible wild plants against nitrosamine precursors experimentally-induced testicular toxicity in male albino rats. J Egypt Soc Toxicol. 2008;38(4):1-11.
-
17.
Mulabagal V, Wang H, Ngouajio M, Nair MG. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur Food Res Technol. 2009;230(1):47-53. https://doi.org/10.1007/s00217-009-1144-7.
-
18.
Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol. 2014;72:138-46. [PubMed ID: 25010453]. https://doi.org/10.1016/j.fct.2014.06.029.
-
19.
Jamshidzadeh A, Khoshnood MJ, Dehghani Z, Niknahad H. Hepatoprotective activity of Cichorium intybus L. leaves extract against carbon tetrachloride induced toxicity. Iran J Pharm Res. 2010;1:41-6.
-
20.
Drummond GS, Kappas A. An experimental model of postnatal jaundice in the suckling rat. Suppression of induced hyperbilirubinemia by Sn-protoporphyrin. J Clin Invest. 1984;74(1):142-9. [PubMed ID: 6547455]. [PubMed Central ID: PMC425194]. https://doi.org/10.1172/JCI111394.
-
21.
Jendrassik L. Colorimetric determination of bilirubin. Biochem. 1938;97:72-81.
-
22.
Bais HP, Ravishankar GA. Cichorium intybus L - cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J Sci Food Agric. 2001;81(5):467-84. https://doi.org/10.1002/jsfa.817.
-
23.
Judžentienė, A, Būdienė, J. Volatile constituents from aerial parts and roots of Cichorium intybus L.(chicory) grown in Lithuania. Chemija. 2008;19(2):25-8.
-
24.
Street RA, Sidana J, Prinsloo G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid Based Complement Alternat Med. 2013;2013:579319. [PubMed ID: 24379887]. [PubMed Central ID: PMC3860133]. https://doi.org/10.1155/2013/579319.
-
25.
Miraldi E, Ferri S, Mostaghimi V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran). J Ethnopharmacol. 2001;75(2-3):77-87. [PubMed ID: 11297838]. https://doi.org/10.1016/S0378-8741(00)00381-0.
-
26.
Sezik E, Yesilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol. 2001;75(2-3):95-115. [PubMed ID: 11297840]. https://doi.org/10.1016/S0378-8741(00)00399-8.
-
27.
Ahmed B, Al-Howiriny TA, Siddiqui AB. Antihepatotoxic activity of seeds of Cichorium intybus. J Ethnopharmacol. 2003;87(2-3):237-40. [PubMed ID: 12860315]. https://doi.org/10.1016/S0378-8741(03)00145-4.
-
28.
Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27(1):1-93. [PubMed ID: 16105678]. https://doi.org/10.1016/j.mam.2005.07.008.
-
29.
Kalantari H, Kooshapur H, Rezaii FRN, Moosavi M. Study of the protective effect of raphanus sativus (radish) seed in liver toxicity induced by carbon tetrachloride in mice. Jundishapur J Nat Pharm Prod. 2009;4(1):24-31.
-
30.
Mirshahvalad S, Feizi F, Barkhordar A, Bahadoram M, Houshmand G, Pouramir M. Hepatoprotective effects of arbutin against liver damage induced by carbon tetrachloride in rats. Jundishapur J Nat Pharm Prod. 2016;11(3). https://doi.org/10.17795/jjnpp.33392.
-
31.
Hajiani E, Jalal Hashemi S. Comparison of therapeutic effects of silymarin and vitamin e in nonalcoholic fatty liver disease: Results of an open-labele, prospective, randomized study. Jundishapur J Nat Pharm Prod. 2009;4(1):8-14.
-
32.
Kalantari H, Khorsandi L, Taherimobarakeh M. The protective effect of the curcuma longa extract on acetaminophen –induced hepatotoxicity in mice. Jundishapur J Nat Pharm Prod. 2007;2(1):7-12.
-
33.
Zafar R, Mujahid Ali S. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J Ethnopharmacol. 1998;63(3):227-31. [PubMed ID: 10030727]. https://doi.org/10.1016/S0378-8741(98)00087-7.
-
34.
Hassan HA, Yousef MI. Ameliorating effect of chicory (Cichorium intybus L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem Toxicol. 2010;48(8-9):2163-9. [PubMed ID: 20478349]. https://doi.org/10.1016/j.fct.2010.05.023.
-
35.
Schmidt BM, Ilic N, Poulev A, Raskin I. Toxicological evaluation of a chicory root extract. Food Chem Toxicol. 2007;45(7):1131-9. [PubMed ID: 17306431]. [PubMed Central ID: PMC3836359]. https://doi.org/10.1016/j.fct.2006.12.019.
-
36.
Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol. 2008;46(10):3325-32. [PubMed ID: 18768152]. https://doi.org/10.1016/j.fct.2008.08.004.
-
37.
Upur H, Amat N, Blazekovic B, Talip A. Protective effect of Cichorium glandulosum root extract on carbon tetrachloride-induced and galactosamine-induced hepatotoxicity in mice. Food Chem Toxicol. 2009;47(8):2022-30. [PubMed ID: 19477217]. https://doi.org/10.1016/j.fct.2009.05.022.
-
38.
Olsen NJ, Branch VK, Jonnala G, Seskar M, Cooper M. Phase 1, placebo-controlled, dose escalation trial of chicory root extract in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2010;11:156. [PubMed ID: 20618964]. [PubMed Central ID: PMC2912794]. https://doi.org/10.1186/1471-2474-11-156.
-
39.
Schumacher E, Vigh E, Molnar V, Kenyeres P, Feher G, Kesmarky G, et al. Thrombosis preventive potential of chicory coffee consumption: a clinical study. Phytother Res. 2011;25(5):744-8. [PubMed ID: 21425378]. https://doi.org/10.1002/ptr.3481.
-
40.
Bernstein RB, Novy MJ, Piasecki GJ, Lester R, Jackson BT. Bilirubin metabolism in the fetus. J Clin Invest. 1969;48(9):1678-88. [PubMed ID: 4980771]. [PubMed Central ID: PMC535739]. https://doi.org/10.1172/JCI106133.
-
41.
Kamisako T, Kobayashi Y, Takeuchi K, Ishihara T, Higuchi K, Tanaka Y, et al. Recent advances in bilirubin metabolism research: the molecular mechanism of hepatocyte bilirubin transport and its clinical relevance. J Gastroenterol. 2000;35(9):659-64. [PubMed ID: 11023036]. https://doi.org/10.1007/s005350070044.
-
42.
Berk PD, Wolkoff AW, Berlin NI. Inborn errors of bilirubin metabolism. Med Clin North Am. 1975;59(4):803-16. [PubMed ID: 806753]. https://doi.org/10.1016/S0025-7125(16)31976-9.
-
43.
Ives NK. Management of neonatal jaundice. Paediatr Child Health. 2015;25(6):276-81. https://doi.org/10.1016/j.paed.2015.02.008.
