Abstract
Background:
QT prolongation is a problem that increases the ventricular arrhythmias in the cirrhotic patients. Sour cherry seed extract has been reported to have potential anti-inflammatory and antioxidant activity.Objectives:
This study aimed to find the effect of Prunus cerasus (sour cherry) kernel seed extract on QT interval of heart and its histopathological parameters in rats with biliary cirrhosis induced by bile duct ligation (BDL).Materials and Methods:
Thirty-two male Sprague Dawley rats (200 - 250 g) were divided into 4 groups; negative control, cirrhotic, and 2 cirrhotic groups treated with sour cherry kernel extract in doses of 10 mg/kg and 30 mg/kg orally for 4 weeks. In all groups before BDL and 4 weeks after surgery animals were anesthetized and their electrocardiograms were recorded. After the required period of BDL, liver was isolated and immersed in 10% formalin solution and stained by hematoxylin and eosin for histological studies.Results:
Results were analyzed by using Student t-test and one-way ANOVA. P < 0.05 was considered as significant level. The QT interval was calculated using the Bazettʼs formula and corrected QT (QTc) was obtained. Four weeks after BDL, the QTc and amount of total and direct bilirubin in cirrhotic group were found as 213.6 ± 6.8 ms, 10.5 ± 1.02 mg/dL, and 7.9 ± 0.7 mg/dL, respectively. These values significantly increased compared to the negative control group (119.9 ± 6.2 ms, 0.11 ± 0.00102 mg/dL, and 0.1 ± 0.0002 mg/dL) (P < 0.001). Histopathological parameters were reduced in cirrhotic groups treated with extract compared to control cirrhotic group.Conclusions:
These results revealed that cirrhosis produce prolonged QT interval and sour cherry extract has an ameliorating effect on QT prolongation probably via its antiarrhythmic property.Keywords
Sour Cheery Cirrhosis QT Interval Histopathological Parameters Rat
1. Background
Liver plays an important and vital role in the body as it participates in the metabolism of carbohydrates, fats, storage of vitamin A, detoxification of toxic substances, production of clotting factors, and so on (1).
Liver cirrhosis is a frequent consequence of the long clinical course of chronic liver diseases. It is characterized by tissue fibrosis and conversion of normal liver architecture into abnormal nodules. Portal hypertension is the most important and earliest symptoms of cirrhosis and underlies most of the clinical complications of the disease (2).
Liver cirrhosis is associated with some hemodynamic changes and is an outcome of chronic liver diseases leading to cardiovascular abnormalities (3-5).
Kowalski et al. reported for the first time that cirrhotic patients had abnormal cardiovascular function and prolonged QT interval (6). The systemic circulation in cirrhotic subjects is hyperdynamic and characterized by increased heart rate (HR), cardiac output, and decreased systemic vascular resistance or arterial blood pressure (3, 7, 8).
QT interval duration can be determined by ECG recording from the onset of the QRS complex to the end of the T wave (8). The QT represents the duration of the ventricular electric systole and its prolongation predisposes ventricular arrhythmias. QT interval varies with heart rate, so that it shortens when heart rate increases. The most frequently used formula to correct QT for heart rate (QTc) has been proposed by Bazett (9, 10).
Equation 1. Bazett’s Formula
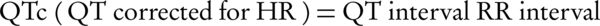
Liver diseases are critical health problems, and curative plants and herbs have been used for healing them all over the world. Due to their safety and efficacy, herbal medicines are utilized increasingly to treat an extensive variety of diseases (11-13).
Sour cherry has polyphenols and flavonoids in seed kernel extracted of its solid fraction, which may have antioxidant effects (14).
It has been reported that orally administered extract of sour cherry rich in flavonoids protect rats against the ischemia reperfusion and improve recovery of cardiac functions, aortic and coronary flow, and left ventricular pressure that reflect in decreased infract size in isolated rat hearts subjected to ischemic reperfusion (15).
2. Objectives
This study aimed to find out the effect of hydroalcoholic sour cherry kernel seed extract on electrophysiology of heart, serum bilirubin, and pathological parameters on a model of induced cholestasis in rats.
3. Materials and Methods
3.1. Plant Material
Solid fraction of sour cherry kernel seed was obtained from the Pharmacology Department of School of Pharmacy of Debrecen Medical University. The solid kernel fraction was soaked in 70% ethanol for 3 days with occasional shaking. The extract was filtered through a clean cotton cloth then solvent was evaporated under vacuum using a rotary evaporator at 40°C (15).
3.2. Chemicals
Ketamine and xylazine were purchased from Alfasan Co (Holland). Hematoxylin and eosin were purchased from Sigma (St. Louis, MO, USA).
3.3. Animals
Male Sprague Dawley rats (200 - 250 g) were obtained from the Animal House of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals were kept under standard laboratory care (temperature 25 ± 2ºC, 12:12 hour light: dark cycle) with access to food and water ad libitum. The protocol implementation was approved by Research Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The animals were randomly divided into 4 groups (negative control [sham], positive control [cirrhotic], and 2 cirrhotic groups treated with 2 doses of extract) and each group consisted of 8 rats.
Extra hepatic cholestasis (biliary cirrhosis) was induced by long-term (4 weeks) double bile duct ligation (BDL) in animals. Each rat was weighed and anesthetized by intrapritoneal administration of ketamin (50 mg/kg) and xylazine hydrochloride (10 mg/kg) (16). Following a midline abdominal incision closed to the sternum, the common bile duct was exposed; a double ligature was made with 3-0 silk suture, after that, a cut was made between both ligatures. Abdominal muscles and abdominal skin were closed with 3-0 silk suture (17, 18).
The negative control group, after anesthetization, shaved, and the common bile duct was only dissected from the surrounding tissue without blocking the bile duct and was treated with normal saline (extract solvent) 1 mL/kg daily for 4 weeks.
The positive control group (by performing BDL, cirrhotic group) was treated with normal saline (extract solvent) 1 mL/kg daily for 4 weeks (19).
The treated group was divided into subgroups (A and B); one of them received 10 mg/kg and the other one 30 mg/kg of the extract for 4 weeks after bile duct ligation.
Then, the animals were allowed to recover in separate cage with free access to food and water.
3.4. QT Interval Assay
Four weeks (28 days) after the beginning of the experiment or BDL, all animals were anesthetized and lead II electrocardiogram (in order to assess QT interval) were recorded (20). Power lab and BioAmp (AD-Instruments Co., Australia) were used for recorded ECG.
3.5. Bilirubin Analysis
Blood samples were withdrawn from the animals’ hearts to measure serum bilirubin as a marker of cholestasis (21). Total and conjugated bilirubin were measured by colorimetric method using an auto-analyzer (BT3000).
3.6. Sample Staining with Hematoxylin and Eosin
After the required period of BDL, animals were anesthetized, liver was isolated and washed with normal saline and immersed in 10% formalin solution for histological studies. Samples were embedded in paraffin. The sections were made in 5 µm thickness with a microtome and stained by hematoxylin and eosin (22). Then, the slides were examined under a microscope (Olympus BH2-RFCA).
3.7. Statistics
Data were presented as Mean ± SEM. Results were analyzed by using Student t-test and one-way ANOVA. P < 0.05 was considered as significant level.
4. Results
Before BDL, comparison of QTc in different groups did not show a significant difference. Four weeks after BDL, QTc in cirrhotic group significantly increased compared to negative control group (P < 0.001). Also significant differences between two groups with doses of extract-treated 10 mg/kg (P < 0.001) and 30 mg/kg (P < 0.05) and negative control group were observed (Figure 1).
Comparison of QTc (in ms) Among Different Groups According to the Bazettʼs Formula
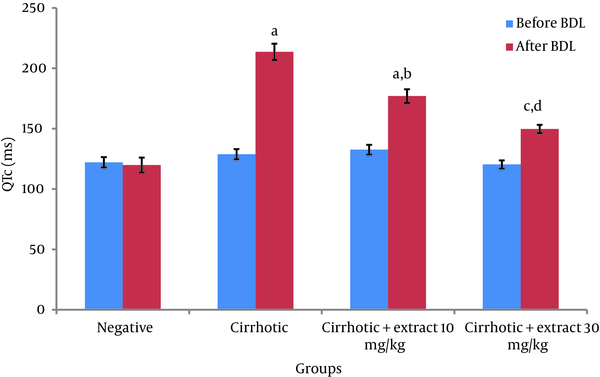
Significantly decreased in QTc in the 10 mg/kg (P < 0.01) and 30 mg/kg (P < 0.001) extract-treated groups was shown in comparison with cirrhotic group. Heart rate did not show any significant difference among all groups before and after BDL (Table 1).
Comparison of Heart Rate Among Different Groups
| Animal Groups | HR, bpm | |
|---|---|---|
| Before BDL | After BDL | |
| Negative | 236.5 ± 5.3 | 242.3 ± 17.0 |
| Cirrhotic | 229 ± 11.5 | 237.7 ± 10 |
| Cirrhotic + 10 mg/kg extract | 256.3 ± 17 | 206.4 ± 11 |
| Cirrhotic + 30 mg/kg extract | 223.1 ± 11 | 212.6 ± 7.6 |
The conjugated and total bilirubin levels in positive control group and 2 groups of extract treated were significantly higher than those levels in negative control group (P < 0.001). Significant difference between the conjugated bilirubin level in cirrhotic treated rats with extract (10 and 30 mg/kg/day) groups was observed compared to cirrhotic group (P < 0.01). There was also significant difference between the total bilirubin level in cirrhotic treated with extract (10 and 30 mg/kg/day) groups and cirrhotic group (P < 0.05) (Table 2).
Comparison of Bilirubin Levels in Different Groups
Results of histopathological samples of liver, stained with hematoxylin, and eosin are shown in Figures 2 - 4 at 40X magnification.
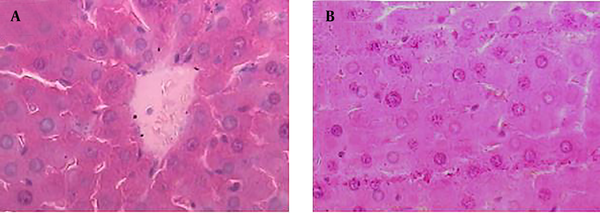
Hematoxylin and Eosin Histopathological Stained of the Liver Tissue (×400) in Negative Control Group
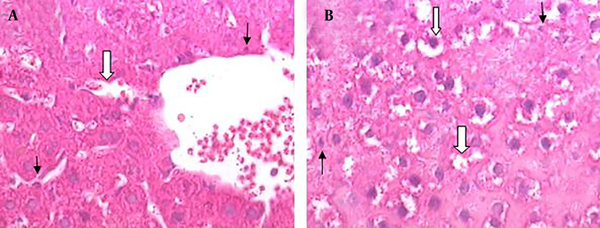
Hematoxylin and Eosin Histopathological Stained of the Liver Tissue (× 400) in Cirrhotic Group
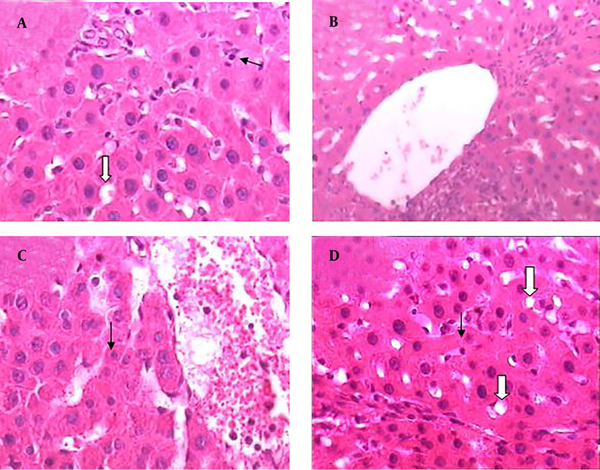
Hematoxylin and Eosin Histopathological Stained of the Liver Tissue (× 400)
The samples from negative control group that received normal saline did not show histopathological changes and normal hepatic parenchyma and hepatocytes were observed (Figure 2 A and B).
The liver morphological changes induced by BDL are shown in Figure 3. Severe histopathological changes were observed in livers of all rats from positive control groups (cirrhotic group). The predominant lesions were extensive degeneration and necrotic cells. The histological appearance of the liver showed cytoplasmic vacuolation that indicated fatty change (Figure 3 A and B).
Treatment by extract (10 and 30 mg/kg/day) ameliorated the changes induced by BDL. The lesions in the liver of cirrhotic rats that received extract (10 and 30 mg/kg/day) were considerably less than those in the cirrhotic rats (Figure 4 A - D).
5. Discussion
In this study, the effect of sour cherry seed kernel extract was studied on QT interval, serum bilirubin, and histopathological parameters of a model of induced cholestasis in rats. Sour cherry seed kernel extract has shown a reduction effect on QT interval, bilirubin levels, and histopathological changes when administrated for 4 weeks to rats suffering from controlled hepatic complications caused by cirrhosis.
A well-known model to create cirrhosis in animals is BDL. It induces cirrhosis in animals which reflects by a significant increase in serum total bilirubin levels (17). In the present study, serum bilirubin level has changed by sour cherry seed kernel extract treatment.
Previous studies had shown that the liver damage markers were elevated bye BDL induced cirrhosis. Chronic BDL significantly increases most of plasma and hepatic cytokine levels (21). Several studies have shown that treatment with free radical scavengers remarkably decreases liver damage following BDL in rats (23). Other results have demonstrated that oxidative stress associated with lipid peroxidation is involved in the development of liver damage in cholestatic rats by BDL (24). This study has shown that histopathological parameters were reduced in cirrhotic groups treated with two doses of extract compared to positive control group.
To understand the potential effect of any unknown compound or substance on body, it is essential to know the mechanism of action of the compound.
Prolongation of QT interval mechanism in the cirrhotic rat is poorly known, therefore the effect of sour cherry seed kernel extract was studied on electrophysiology of heart in rats with billary cirrhosis induced by BDL. In our previous research, we reported that sour cherry has potential antioxidant, anti-inflammatory, and free radical scavenger effects (25), thus we tried to find out to what extent this compound can influence QT interval by a well-known method.
The prolongation of QT interval is one of the important symptoms of cardiac abnormality in cirrhotic patients with ventricular arrhythmia risk (6).
Sour cherry seed extract as a source of antioxidant at different doses were administered to cirrhotic rats. Significant decrease in QT interval, laboratory, and histopathological parameters in BDL group treated by sour cherry seed extract suggest the protective effect of this extract in cirrhotic patients.
Acknowledgements
References
-
1.
Kuntz E, Kuntz HD. Hepatology, Principles and practice: history, morphology, biochemistry, diagnostics, clinic, therapy. Berlin, Heidelberg: Springer Science & Business Media; 2006. p. 31-71.
-
2.
Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25(2):281-90. [PubMed ID: 21497745]. https://doi.org/10.1016/j.bpg.2011.02.009.
-
3.
Pudil R, Pelouch R, Praus R, Vasatova M, Hulek P. Heart failure in patients with liver cirrhosis. Cor et Vasa. https://doi.org/10.1016/j.crvasa.2013.06.002.
-
4.
Lee SS. Cardiac abnormalities in liver cirrhosis. West J Med. 1989;151(5):530-5. [PubMed ID: 2690463].
-
5.
Lee RF, Glenn TK, Lee SS. Cardiac dysfunction in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21(1):125-40. [PubMed ID: 17223501]. https://doi.org/10.1016/j.bpg.2006.06.003.
-
6.
Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec's cirrhosis. J Clin Invest. 1953;32(10):1025-33. [PubMed ID: 13096569]. https://doi.org/10.1172/JCI102813.
-
7.
Llach J, Gines P, Arroyo V, Rimola A, Tito L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94(2):482-7. [PubMed ID: 3335320].
-
8.
Moller S, Wiinberg N, Hernriksen JH. Noninvasive 24-hour ambulatory arterial blood pressure monitoring in cirrhosis. Hepatology. 1995;22(1):88-95. [PubMed ID: 7601438].
-
9.
Zambruni A, Trevisani F, Caraceni P, Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol. 2006;44(5):994-1002. [PubMed ID: 16510203]. https://doi.org/10.1016/j.jhep.2005.10.034.
-
10.
Torregrosa M, Aguade S, Dos L, Segura R, Gonzalez A, Evangelista A, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42(1):68-74. [PubMed ID: 15629509]. https://doi.org/10.1016/j.jhep.2004.09.008.
-
11.
Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci. 2005;50(10):1807-12. [PubMed ID: 16187178]. https://doi.org/10.1007/s10620-005-2942-9.
-
12.
Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39(4):293-304. [PubMed ID: 17331820]. https://doi.org/10.1016/j.dld.2006.11.004.
-
13.
Saleem TM, Chetty CM, Ramkanth S, Rajan V, Kumar KM, Gauthaman K. Hepatoprotective herbs-a review. Int J Res Pharm Sci. 2010;1(1):1-5.
-
14.
Piccolella S, Fiorentino A, Pacifico S, D'Abrosca B, Uzzo P, Monaco P. Antioxidant properties of sour cherries (Prunus cerasus L.): role of colorless phytochemicals from the methanolic extract of ripe fruits. J Agric Food Chem. 2008;56(6):1928-35. [PubMed ID: 18303821]. https://doi.org/10.1021/jf0734727.
-
15.
Bak I, Lekli I, Juhasz B, Nagy N, Varga E, Varadi J, et al. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am J Physiol Heart Circ Physiol. 2006;291(3):H1329-36. [PubMed ID: 16617126]. https://doi.org/10.1152/ajpheart.01243.2005.
-
16.
Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274(3 Pt 2):H747-51. [PubMed ID: 9530184].
-
17.
Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112(11):1678-87. [PubMed ID: 14623915]. https://doi.org/10.1172/JCI18945.
-
18.
Rivera-Huizar S, Rincon-Sanchez AR, Covarrubias-Pinedo A, Islas-Carbajal MC, Gabriel-Ortiz G, Pedraza-Chaverri J, et al. Renal dysfunction as a consequence of acute liver damage by bile duct ligation in cirrhotic rats. Exp Toxicol Pathol. 2006;58(2-3):185-95. [PubMed ID: 16829063]. https://doi.org/10.1016/j.etp.2006.05.001.
-
19.
Payabvash S, Kiumehr S, Nezami BG, Zandieh A, Anvari P, Tavangar SM, et al. Endogenous opioids modulate hepatocyte apoptosis in a rat model of chronic cholestasis: the role of oxidative stress. Liver Int. 2007;27(4):538-47. [PubMed ID: 17403194]. https://doi.org/10.1111/j.1478-3231.2007.01457.x.
-
20.
Lanjewar P, Pathak V, Lokhandwala Y. Issues in QT interval measurement. Indian Pacing Electrophysiol J. 2004;4(4):156-61. [PubMed ID: 16943929].
-
21.
Fernandez-Martinez E, Perez-Alvarez V, Tsutsumi V, Shibayama M, Muriel P. Chronic bile duct obstruction induces changes in plasma and hepatic levels of cytokines and nitric oxide in the rat. Exp Toxicol Pathol. 2006;58(1):49-58. [PubMed ID: 16617007]. https://doi.org/10.1016/j.etp.2006.03.002.
-
22.
Olteanu D, Nagy A, Dudea M, Filip A, Muresan A, Catoi C, et al. Hepatic and systemic effects of rosuvastatin on an experimental model of bile duct ligation in rats. J Physiol Pharmacol. 2012;63(5):483-96. [PubMed ID: 23211302].
-
23.
Dianat M, Fard SS, Badavi M, Ahangarpour A. The effect of caffeic acid phenethyl ester on QT interval in cirrhotic rats. Healthmed. 2012;6(10):3281-5.
-
24.
Coban S, Yildiz F, Terzi A, Al B, Ozgor D, Ara C, et al. The effect of caffeic acid phenethyl ester (CAPE) against cholestatic liver injury in rats. J Surg Res. 2010;159(2):674-9. [PubMed ID: 19535096]. https://doi.org/10.1016/j.jss.2008.10.023.
-
25.
Bak I, Czompa A, Csepanyi E, Juhasz B, Kalantari H, Najm K, et al. Evaluation of systemic and dermal toxicity and dermal photoprotection by sour cherry kernels. Phytother Res. 2011;25(11):1714-20. [PubMed ID: 21751269]. https://doi.org/10.1002/ptr.3580.