Abstract
Background:
In general, seasonal growing of plants influences chemical composition and biological activities of essential oils.Objectives:
Therefore, the aerial parts of Scrophularia frigida (S. frigida) were used in the current study to find seasonal variations in the content and composition of essential oil.Materials and Methods:
The oil was extracted by hydro-distillation from two samples collected in different seasons, and analyzed by GC-FID and GC-MS.Results:
In total, 18 and 20 components were identified and quantified in the oil of summer and autumn samples representing 97.42% and 94.77% of the oil components, respectively. The essential oil of autumn sample was mainly composed of fatty acid derivatives (42.69%). The major components of the autumn oil were palmitic acid (30.49%), phytol (12.99%), L-linalool (11.41%), and hexahydrofarnesyl acetone (6.65%). The essential oil of summer sample was dominated by oxygenated monoterpenes (68.91%), L-linalool (38.69%), geraniol (11.20%), α-terpineol (9.99%), and palmitic acid (7.32%).Conclusions:
Based on these observations, wide variation is detected in the chemical composition of the oil obtained from the same plant in different seasons.Keywords
1. Background
In recent decades, there has been a great deal of scientific interest in the use of essential oils and plant extracts due to their natural antioxidants and biologically potent metabolites. The European Union countries enormously use essential oils in food industry (as flavors and preservatives), perfumes (fragrances), and pharmaceuticals (for their functional properties) (1). The genus Scrophularia is one of the largest genera of the Scrophulariaceae family, comprising about 300 species (2), mostly represented by perennial herbs, distributed in the Central Europe, Asia, North America, and northern hemisphere, especially in the Mediterranean area (3). In Iran, 42 species are reported; 19 of them are considered as endemic taxa (2, 3). Among them, S. frigida, with its Persian name “Gole Meimoonye Yakhchaali”, is a perennial herb with erect glabrous square stems up to 80 cm. leaves are opposite, rarely upper leaves alternate, glabrous and ovate or ovate-lanceolate, highest ones angust linear; inflorescences are cyme-raceme with open two-lipped flowers (4). Various biological compounds such as phenolic acids, iridoids, phenylpropanoids, flavonoids, saponins, and terpenoids have been isolated from this genus (5-10). These compounds possess anti-inflammatory, antibacterial, immunomodulatory, cardiovascular, diuretic, molluscicidal, cytotoxic, and antitumor properties (11-15). In traditional Chinese medicine, extract of S. ningpoensis Hemsl has been used in the treatment of fever, constipation, pharyngitis, and neuritis (16). Although there are several species of Scrophularia growing in different parts of Iran, few studies have been conducted on the chemical composition of its essential oil (17-20).
2. Objectives
To the best of our knowledge, there is no previous report about chemical composition of essential oil of S. frigida. Therefore, the first aim of this study was to elucidate the composition of this plant essential oil using a combination of GC-FID and GC-MS, and the second aim was to determine seasonal variations (summer vs. autumn) in the composition of its essential oil.
3. Materials and Methods
3.1. Plant Material
Aerial parts of S. frigida were collected from Misho-dagh Mountain near Marand City (Yam) in East Azerbaijan province, Iran, during different seasons (June and October). The collected parts of S. frigida were transferred to Herbarium of Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran, and botanically confirmed by herbalist. Voucher specimens have been deposited with the Herbarium under accession code TBZ-Fph-746.
3.2. Isolation of Essential Oil
The air-dried aerial parts of S. frigida (100 g) were cut into small pieces and submitted to hydro-distillation for 4 hours in a clevenger apparatus using hexane (2 mL) as collector solvent. The pale yellow-colored essential oil were dried (under Na2SO4) and stored in sealed vials. The oil yield (0.06% w/w for summer oil and 0.04% w/w for autumn oil) was estimated on the dry-weight basis.
3.3. GC-MS Analysis
About 1µL of volatile oil/hexane solution was injected into GC-MS analyzer using a Shimadzu capillary GCMS-QP 5050A gas chromatograph mass spectrometer, and DB1 capillary column (60 m x 0.25 mm, I.D., film thickness 0.25 µm) using He as carrier gas with flow rate 1mL/min along with split ratio of 1.29, which was equipped with flame ionization detector (FID). Condition of GC was as follows: The range of column temperature started at 60°C for 5 minutes then maintained at 60°C, -230°C for 20 minutes, finally holding 3 minutes at 280°C. Injector and detector temperature was 220°C and 230°C, respectively. Moreover, all mass spectra were recorded in electron-impact mode with an ionization voltage of 70 eV. Ion source temperature and quadrupole were 270°C and 100°C, respectively. Other parameters were considered as: solvent delay 2 minutes; scan speed 2000 amu/s; scan range 30 - 600 amu, and eV voltage 3000 V.
3.4. Identification of Compounds
The identification of volatile aroma compounds was based on the Kovats indices (KI) on DB1 column which was calculated with reference to homologues series of n-alkans C8 - C20. Also the peak identification was confirmed by computer matching with the Wiley 229, NIST107 along with other published fragmentation patterns of the mass spectra (20). Furthermore, FID response was applied for determining the relative amounts of each constituent, without using correction factors.
4. Results
Two samples of the aerial parts of S. frigida were collected during summer and autumn (June and October 2013) from plants in one site. The chemical composition of the essential oils collected in the two seasons was qualitatively and quantitatively investigated by GC-MS and GC-FID, respectively. The oil content in S. frigida during summer (600 mg/kg) was relatively higher than the sample collected during autumn (400 mg/kg). The composition of the essential oils, the percentage of individual constituents and the Kovats indexes are presented in the Table 1. In total, 20 and 18 compounds were identified and quantified in the oils of autumn and summer accounting for 94.77% and 97.24% of the oils amount, respectively. The data for the current study demonstrated that the contents of the essential oils were varied remarkably with regard to seasonal changes.
The Chemical Composition of S. frigida Essential Oil During Two Different Seasons
| Compound a | KI | Summer, % | Autumn, % | Identification Method b |
|---|---|---|---|---|
| Tropilidin | 785 | NA | 1.26 | GC/MS, Ib |
| 1-Methylcyclopentanol | 951 | NA | 1.72 | GC/MS, Ib |
| 1-Octen-3-ol | 963 | 7.27 | 0.7 | GC/MS, Is |
| n-Nonaldehyde | 1083 | 1.95 | 0.55 | GC/MS, Is |
| L-linalool | 1086 | 38.69 | 11.41 | GC/MS, Is |
| α-Terpineol | 1174 | 9.99 | NA | GC/MS, Is |
| Linalyl propionate | 1176 | NA | 4.62 | GC/MS, Is |
| Decanal | 1185 | 0.77 | NA | GC/MS, Is |
| Nerol | 1210 | 3.49 | 6.46 | GC/MS, Is |
| E-Geraniol | 1235 | 11.20 | NA | GC/MS, Is |
| E-2-decenal | 1238 | 0.54 | NA | GC/MS, Is |
| β-Hydroxylauric acid | 1252 | 1.00 | NA | GC/MS, Is |
| Carvacrol | 1278 | 4.23 | NA | GC/MS, Is |
| Damascenone | 1364 | 1.72 | 1.06 | GC/MS, Is |
| Spathulenol | 1371 | 1.10 | 0.6 | GC/MS, Is |
| β-Copaen-4 -α-ol | 1374 | NA | 1.64 | GC/MS, Is |
| E-β-Ionone | 1466 | 0.92 | NA | GC/MS, Is |
| E-Nerolidol | 1549 | 2.01 | 2.38 | GC/MS, Is |
| Octadecanal | 1696 | 0.54 | NA | GC/MS, Is |
| Myristic acid | 1741 | NA | 1.26 | GC/MS, Is |
| Farnesyl acetate | 1817 | NA | 1.3 | GC/MS, Is |
| Hexahydrofarnesyl acetone | 1831 | 1.85 | 6.65 | GC/MS, Is |
| N-Hexadecanoic acid | 1943 | 7.32 | 30.49 | GC/MS, Is |
| Methyl 9,12,15-octadecatrienoate | 2085 | NA | 0.68 | GC/MS, Ib |
| Heneicosane | 2100 | NA | 3.36 | GC/MS, Ib |
| Oleic acid | 2116 | NA | 3.81 | GC/MS, Ib |
| Phytol | 2124 | 2.65 | 12.99 | GC/MS, Ib |
| Hexadecanal diallyl acetal | 2286 | NA | 1.83 | GC/MS, Ib |
| Total compounds | NA | 97.24 | 94.77 | |
| Non-terpenoid | NA | 22.57 | 59.29 | |
| Fatty acids derivatives | 8.32 | 42.69 | ||
| Ketones and aldehydes | 6.98 | 9.56 | ||
| Others | 7.27 | 7.04 | ||
| Terpenoids | NA | 74.67 | 35.48 | |
| Oxygenated monoterpenes | 68.91 | 17.87 | ||
| Oxygenated sesquiterpenes | 3.11 | 4.62 | ||
| Diterpenoids | 2.65 | 12.99 |
5. Discussion
As depicted in Figure 1, in the case of summer oil, monoterpens (68.91%) were represented mainly by oxygenated compounds such as alcohols with L-linalool (38.69%), geraniol (11.20%), and α-terpineol (9.99%), while the contents of fatty acid derivatives was rather low (8.32%). This finding is in line with the observations of Pasdaran et al. (17) and Amiri et al. (18) about essential oil of Scrophularia genus with the same harvesting season. Conversely, in the case of autumn oil, non-terpenoid compounds made up the higher contribution (59.29%) with fatty acids dominating (42.69%) while the content of monoterpenes was amounted to 17.87%. Fatty acids were represented mainly by n-hexadecanoic acid or palmitic acid (30.49%), and to a lesser extent, by oleic acid (3.81%) and myristic acid (1.26%) in autumn essential oil. The abundance of palmitic acid in S. frigida is consistent with that reported for other representatives of Scrophularia genus such as S. ningpoensis (19). This oil also contained high amount of diterpenes (12.99%), represented by (E)-phytol, in comparison with summer oil (2.65%) (Table 1 and Figure 1).
Seasonal Variation in the Major Chemical Groups of S. frigida Essential Oils
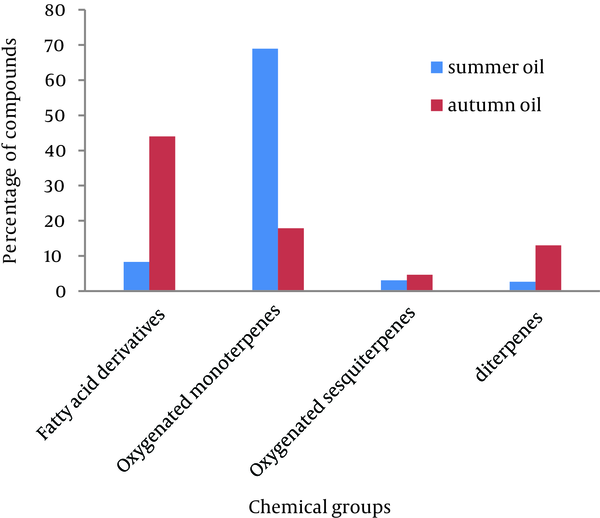
Presence of phytol as one of the main components in autumn oil is in agreement with the findings of Pasdaran et al. about essential oil from aerial parts of S. oxycephala (20). However, the percentage of 1-octen-3-ol (0.7%) was quite lower in autumn oil compared with that of summer oil (7.2%). Previous studies exhibited that the presence of 1-octen-3-ol (an aliphatic alcohol) may be resulted from a simultaneous loss of water-soluble components and an increase in oxidation products during drying process (21). Conversely, hexahydrofarnesyl acetone (6, 10, 14-trimethyl-2-pentadecanone) was present at high level in autumn oil (6.65%) compared with that of summer oil (1.85%). These compounds were routinely seen in essential oils of other species of Scrophularia (17-20). The variations in the chemical composition of these essential oils may be attributed to climatic conditions, harvesting season, and developmental stage (22). In the light of above findings, there might be a reciprocal regulation between oxygenated monoterpenes and fatty acid derivatives. Various types of terpenes are involved in protection against photooxidative stress, attraction of pollinators, moderating thermotolerance, and direct protection against insects and microbes. Moreover, terpene metabolites are vital for plant growth and development and also interacting between plants and their environments (23-25). Therefore, the high amount of oxygenated monoterpenes in summer oil might have been due to these reasons. Our data confirm that environmental conditions and seasonal variations strongly impact on metabolism of monoterpenes. Notably, in our examined oils, no aromatic compounds were detected while the presence of these types of compounds such as eugenol, eugenol acetate, anethol, and so on is common in Scrophularia essential oils (17, 19, 20). In summary, the present study for the first time reported the chemical composition of the essential oils from aerial parts of S. frigida collected in different seasons. The result also indicated that the essential oil yield and composition is affected by intrinsic parameters such as developmental stages of the plant and extrinsic ones like temperature and humidity conditions.
Acknowledgements
References
-
1.
Burt S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol. 2004;94(3):223-53. [PubMed ID: 15246235]. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022.
-
2.
Mozaffarian V. A Dictionary of Iranian Plant Names. 1996.
-
3.
Ardeshiry Lajimi A, Rezaie-Tavirani M, Mortazavi SA, Barzegar M, Moghadamnia SH, Rezaee MB. Study of Anti Cancer Property of Scrophularia striata Extract on the Human Astrocytoma Cell Line (1321). Iran J Pharm Res. 2010;9(4):403-10. [PubMed ID: 24381605].
-
4.
Rechinger KH. Flora Iranica. Graze-Austria: Akad. Druck. U .verlagsanstalt; 1981.
-
5.
Fernandez MA, Garcia MD, Saenz MT. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J Ethnopharmacol. 1996;53(1):11-4. [PubMed ID: 8807471].
-
6.
Ghisalberti EL. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine. 1998;5(2):147-63. [PubMed ID: 23195768]. https://doi.org/10.1016/S0944-7113(98)80012-3.
-
7.
Stevenson PC, Simmonds MS, Sampson J, Houghton PJ, Grice P. Wound healing activity of acylated iridoid glycosides from Scrophularia nodosa. Phytother Res. 2002;16(1):33-5. [PubMed ID: 11807962].
-
8.
Lacaille-Dubois MA, Wagner H. Importance pharmacologique des dérivés polyphénoliques. Acta botanica gallica. 1996;143(6):555-62. https://doi.org/10.1080/12538078.1996.10515353.
-
9.
Bermejo Benito P, Abad Martinez MJ, Silvan Sen AM, Sanz Gomez A, Fernandez Matellano L, Sanchez Contreras S, et al. In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci. 1998;63(13):1147-56. [PubMed ID: 9763210].
-
10.
Emam AM, Diaz-Lanza AM, Matellano-Fernandez L, Faure R, Moussa AM, Balansard G. Biological activities of buddlejasaponin isolated from Buddleja madagascariensis and Scrophularia scorodonia. Pharmazie. 1997;52(1):76-7. [PubMed ID: 9035239].
-
11.
Diaz AM, Abad MJ, Fernandez L, Silvan AM, De Santos J, Bermejo P. Phenylpropanoid glycosides from Scrophularia scorodonia: in vitro anti-inflammatory activity. Life Sci. 2004;74(20):2515-26. [PubMed ID: 15010262]. https://doi.org/10.1016/j.lfs.2003.10.008.
-
12.
Tasdemir D, Brun R, Franzblau SG, Sezgin Y, Calis I. Evaluation of antiprotozoal and antimycobacterial activities of the resin glycosides and the other metabolites of Scrophularia cryptophila. Phytomedicine. 2008;15(3):209-15. [PubMed ID: 17761408]. https://doi.org/10.1016/j.phymed.2007.07.032.
-
13.
Akdemir Z, Kahraman C, Tatli II, Kupeli Akkol E, Suntar I, Keles H. Bioassay-guided isolation of anti-inflammatory, antinociceptive and wound healer glycosides from the flowers of Verbascum mucronatum Lam. J Ethnopharmacol. 2011;136(3):436-43. [PubMed ID: 20621642]. https://doi.org/10.1016/j.jep.2010.05.059.
-
14.
Shen X, Eichhorn T, Greten HJ, Efferth T. Effects of Scrophularia ningpoensis Hemsl. on Inhibition of Proliferation, Apoptosis Induction and NF-kappaB Signaling of Immortalized and Cancer Cell Lines. Pharmaceuticals (Basel). 2012;5(2):189-208. [PubMed ID: 24288088]. https://doi.org/10.3390/ph5020189.
-
15.
Nishibe S. Bioactive phenolic compounds in traditional medicines. Pure appl chem. 1994;66(10-11):2263-6. https://doi.org/10.1351/pac199466102263.
-
16.
Miyazawa M, Okuno Y, Nakamura SI, Kameoka H. Suppression of SOS-inducing activity of chemical mutagens by cinnamic acid derivatives from Scrophulia ningpoensis in the Salmonella typhimurium TA1535/pSK1002 umu test. J Agr Food Chem. 1998;46(3):904-10. https://doi.org/10.1021/jf9704221.
-
17.
Pasdaran A, Delazar A, Nazemiyeh H, Nahar L, Sarker SD. Chemical composition, and antibacterial (against Staphylococcus aureus) and free-radical-scavenging activities of the essential oils of Scrophularia amplexicaulis Benth. Rec Nat Prod. 2012;6:350-5.
-
18.
Amiri H, Lari Y, Esmaeili A, Samsamnia F, Eghbali D, Viskarami GH, et al. Essential oil composition and anatomical study of Scrophularia striata Boiss. Iranian J Med Aromatic Plants. 2011;27:271-8.
-
19.
Miyazawa M, Okuno Y. Volatile components from the roots of Scrophularia ningpoensis Hemsl. Flavour Fragrance J. 2003;18(5):398-400. https://doi.org/10.1002/ffj.1232.
-
20.
Pasdaran A, Nahar L, Asnaashari S, Sarker SD, Delazar A. GC-MS Analysis, Free-Radical-Scavenging and Insecticidal Activities of Essential Oil of Scrophularia oxysepala Boiss. Pharm Sci. 2013;19(1):1.
-
21.
Maggi F, Martonfi P, Conti F, Cristalli G, Papa F, Sagratini G, et al. Volatile components of whole and different plant parts of bastard balm (Melittis melissophyllum L., Lamiaceae) collected in Central Italy and Slovakia. Chem Biodivers. 2011;8(11):2057-79. [PubMed ID: 22083918]. https://doi.org/10.1002/cbdv.201000365.
-
22.
Mojarrab M, Delazar A, Esnaashari S, Afshar FH. Chemical composition and general toxicity of essential oils extracted from the aerial parts of Artemisia armeniaca Lam. and A. incana (L.) Druce growing in Iran. Res Pharm Sci. 2013;8(1):65-9. [PubMed ID: 24459478].
-
23.
Paolini J, Barboni T, Desjobert JM, Djabou N, Muselli A, Costa J. Chemical composition, intraspecies variation and seasonal variation in essential oils of Calendula arvensis L. Biochem Systematics Ecol. 2010;38(5):865-74. https://doi.org/10.1016/j.bse.2010.07.009.
-
24.
Loreto F, Pinelli P, Manes F, Kollist H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004;24(4):361-7. [PubMed ID: 14757575].
-
25.
Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5(3):237-43. [PubMed ID: 11960742].