Abstract
Background:
Chromium is a naturally occurring heavy metal found in the environment.Objectives:
The aim of the present study was to determine the effect of simvastatin (SIMV) on sodium dichromate [Cr(VI)]-induced oxidative stress in the rat lung.Materials and Methods:
Forty-eight adult male Wistar rats (180 - 220 g weight) were randomly assigned to eight groups (each n = 6). Group one received SIMV 20 mg/kg/day. Group two was given vehicle only. Groups three, five, and seven received intraperitoneal (i.p.) Cr(VI) at doses of 8, 12, and 16 mg/kg body weight, respectively, for eight consecutive days. Groups four, six, and eight were pretreated with 20 mg/kg SIMV 30 minutes prior to administration of Cr(VI) at doses of 8, 12, and 16 mg/kg, respectively, for eight consecutive days. Twenty-four hours after the last administration, the animals were killed with an overdose of sodium pentobarbital. Lung tissues were excised for measurements of malondialdehyde (MDA) and glutathione (GSH), and for histopathological examination.Results:
The level of GSH was significantly decreased in the Cr(VI)-treated rats. In contrast, the lung level of MDA was significantly increased in a dose-dependent manner in the Cr(VI)-treated rats when compared to the control animals. SIMV had no effect on GSH and MDA levels compared to the control rats, but there were significantly increased GSH concentrations and decreased MDA levels in lungs of rats treated with Cr(VI).Conclusions:
The observations suggest that SIMV may have a protective effect against Cr(VI)-induced oxidative stress in the rat lung.Keywords
Simvastatin Sodium Dichromate Malondialdehyde Glutathione Rat
1. Background
Chromium is a naturally occurring heavy metal found in the environment. Hexavalent chromium [sodium dichromate, Cr(VI)] compounds are highly toxic to biological systems, but are widely used in many industries, such as chrome plating, welding, wood processing, and tanneries (1, 2). Cr(VI) enters the body through the lungs and gastrointestinal tract, and to a lesser extent through the skin. Exposure to this agent can lead to severe respiratory, cardiovascular, gastrointestinal, hepatic, and renal damage (3-6). Cytotoxicity and genotoxicity of Cr(VI) in humans has been reported (7). Chronic exposure to high concentrations of Cr(VI) in drinking water causes intestinal adenomas and carcinomas in mice. Cr(VI) produces damage to intestinal villi and crypt hyperplasia in mice after only one week of exposure (3, 4). Kumar and Gangwar (2012) reported that Cr(VI) produced injuries in the liver and kidney (8). Lipid peroxidation (LP) and oxidative stress in the liver and kidney of Cr(VI)-exposed rats has been reported (8). Xiao et al. (2012) found that Cr(VI) also induced liver mitochondrial damage (9).
The respiratory tract is the primary target of inhaled chromium. Exposure to Cr(VI) has induced pulmonary injuries in humans and animals (6, 10-13). Asatiani et al. (2011) reported that Cr(VI) can impair the antioxidant defense system (14).
The mechanism of lung toxicity of Cr(VI) compounds has not been clearly documented; however, chromates are readily taken up by cells and reduced to reactive Cr species, which may also result in the generation of reactive oxygen species (ROS) (14, 15). Cr(VI) is rapidly converted to Cr(III) intracellularly, and can induce apoptosis through different mechanisms (14, 15).
Many compounds have been identified as having antioxidant activity. Each of these antioxidants has specific activities, and they often work synergistically to enhance the overall antioxidant capacity of the body (16). The favorable effects of statins can be expanded to antioxidant, anti-inflammatory, and immunomodulatory effects in an in vivo murine model of allergic asthma (17-20). The antioxidant properties of statins extend to organ protection, especially the myocardium and the lungs. Statins have been shown to both prevent and attenuate pulmonary hypertension in animal models. It has been reported that statins protect the kidneys against gentamicin-, cisplatin-, and cyclosporine-induced nephrotoxicity (19, 21, 22). Simvastatin (SIMV) modulates a number of the underlying processes described in lung injury (18, 20). As Cr(VI) is a toxic heavy metal with wide industrial applications and significant potential for occupational exposure, studying the effects of SIMV on Cr(VI)-induced lung injury in experimental animals may be useful for a better understanding of the clinical picture following Cr(VI) exposure in humans. Such studies may lead to a better understanding of the mechanisms by which Cr(VI) may induce pulmonary toxicity. The purpose of the present study was to study the effect of SIMV on Cr(VI)-induced injury in the rat lung.
2. Objectives
The aim of the present study is to determine the effect of SIMV on Cr(VI)-induced oxidative stress in the rat lung.
3. Materials and Methods
3.1. Chemicals
All reagents and chemicals were of analytical grade or higher purity. Cr(VI) was purchased from Aldrich Chemical Co., thiobarbituric acid (TBA) was purchased from Sigma Chemical Co. Other products included SIMV (Tehran chemise), 5,5-dithiobis,2-nitrobinzoicacid (DTNB) (Sigma), 1,1,3,3-tetraethoxypropane (TEP) (Merck), and trichloroacetic acid (TCA) 50% (ACROS). Reduced glutathione (GSH) and sodium pentobarbital were obtained from Sigma Chemical Co.
3.2. Animal Treatments
This study was conducted using 48 adult male Wistar rats (180 - 220 g). The animals were kept under standard 12/12-hour darkness/brightness conditions at a temperature of 23 ± 2°C. The standard pellet rat diet and tap water were freely available. The rats were divided randomly into eight groups (each n = 6). The study groups were assigned the following regimens: Group one was treated with SIMV via oral gavage at a dose of 20 mg/kg body weight (BW)/day for 8 consecutive days. Group two was used as the control group and given vehicle only (normal saline). Groups three, five, and seven received intraperitoneal (i.p.) Cr(VI) at doses of 8, 12, and 16 mg/kg BW/day, respectively, for eight consecutive days. Groups four, six, and eight were pretreated with SIMV (20 mg/kg BW via oral gavage) 30 minutes prior to administration of Cr(VI) (8, 12, and 16 mg/kg BW, respectively) for eight consecutive days.
Twenty-four hours after the last treatment, all of the rats were killed with an overdose of sodium pentobarbital. Lung tissues were removed and washed with normal saline, then fixed and processed for light microscopy, using hematoxylin and eosin (H&E) staining. Five histological sections, each at least 15 µm thick, were taken from each tissue block and stained with H&E. The criteria for cell injury included nuclear dilation, loss of staining capacity, and obvious cellular swelling. Other parts of the lung tissues were collected for determination of malondialdehyde (MDA) and glutathione (GSH) levels. MDA was estimated by the method of Buege and Aust (23), while GSH concentration was measured with DTNB by Ellman’s method (24).
The study protocol was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences.
In this experiment, the dose of Cr(VI) to induce oxidative stress was based on a report by Goodarzi et al. (25) and Ferreira et al. (26). The selected dose of SIMV was as per Ozbakis-Dengiz et al. (27) and Mohammadi et al. (28).
3.3. Biochemical Assays
3.3.1. Peroxidation Markers
MDA, the product of lipid peroxidation, was estimated by the method described by Buege and Aust (1978). Tissue lipid peroxidation was measured in whole-lung homogenate, and after centrifugation at 10,000 g for 10 minutes, the supernatant was taken. Aliquots (1 mL) were analyzed for MDA content after the addition of 2 mL of thiobarbituric acid reagent. The tube was then vortex-mixed for 10 seconds and placed in a boiling water bath (90 - 100°C) for 20 minutes. After cooling for 7 minutes, the resulting supernatant was removed and measured at a wavelength of 532 nm with a SERIEC-7000 spectrophotometer. The MDA concentration was determined by using TEP as an external standard (0.5 - 2.5 µM) (23).
3.3.2. Estimation of Reduced GSH
Reduced GSH was measured by the method of Ellman (1959). For this, 5 mL aliquots were added to 4 mL of distilled water (DW) and 1 mL of 5% trichloroacetic acid (TCA), and the mixture was vortexed and centrifuged at 3,000 g for 15 minutes. Then, 2 mL of supernatant was added to 4 mL of Tris buffer (0.4 M, pH 8.9) and 0.1 mL of DTNB. The mixture was allowed to stand for approximately 5 min, and forming a yellow substance. The absorbance was measured at 412 nm (24).
3.4. Statistical Analysis
The data were analyzed using SPSS 16.0. One-way analysis of variance (ANOVA) was performed, followed by post hoc analysis with the LSD test. A probability value of ≤ 0.05 was determined to be statistically significant.
4. Results
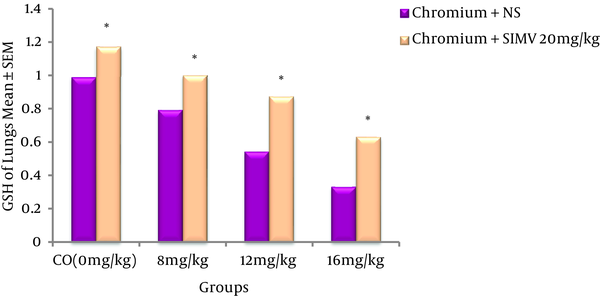
Cr(VI) induced dose-dependent elevation of MDA levels in rat lungs when compared to the control group (P ≤ 0.05). SIMV had no effect on rat lung MDA levels when compared to the control group, but this chemical significantly (P ≤ 0.05) decreased the MDA concentrations in Cr(VI)-treated rats when compared to those receiving the same dose of Cr(VI) only (Figure 1). GSH levels were significantly decreased in the Cr(VI)-treated rats compared to the control animals. However, pre-treatment of animals with SIMV markedly increased the GSH levels in the Cr(VI)-treated rats when compared to the non-pretreated rats that received the same dose of Cr(VI) (Figure 2).
Effect of Simvastatin on MDA Levels in Rat Lung Tissues Treated With Cr(VI) (Chromium)

Effect of Simvastatin on GSH Levels in Lung Tissues of Rats Treated With Cr(VI) (Chromium)
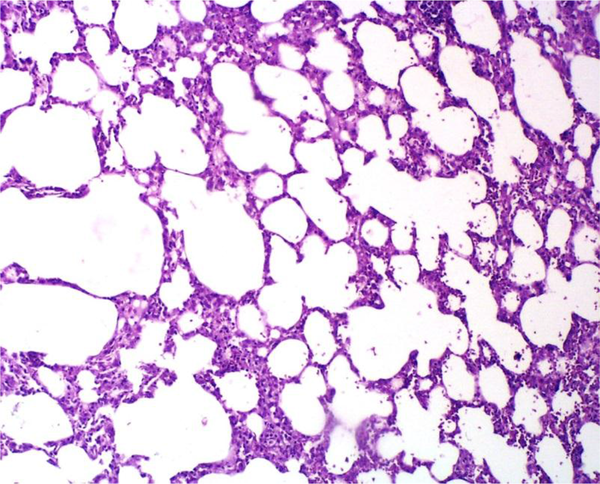
Administration of saline (vehicle) alone did not produce detectable injuries in the rat lungs (Figure 3). However, Cr(VI)-induced damage in the lung tissue included marked infiltration of inflammatory cells into the alveolar space and septal thickening (Figure 4). The extent of injury was increased in a dose-dependent manner. SIMV caused no obvious injury in the rat lungs, with the lung tissue in these rats similar to that of the control animals. However, SIMV markedly reduced pulmonary damage in the Cr(VI)-treated rats (Figure 5).
Light Micrograph of Lung of a Control Rat, Showing no Obvious Injury to the Lung Cells (H&E 10×)
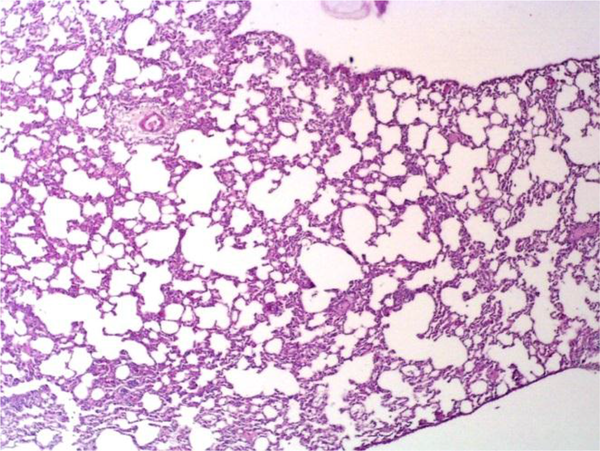
Light Micrograph of a Rat Lung Treated With 16 mg/kg Cr(VI), Showing Extensive Injury, Including Marked Infiltration of Inflammatory Cells Into the Alveolar Space and Septal Thickening (H&E 20×)

Light Micrograph of rat Lung Pretreated With 20 mg/kg SIMV and Treated With 16 mg/kg of Cr(VI), Showing Cr(VI)-Induced Lung Injury That was Diminished Compared to the Non-Pretreated Animals That Received the Same SIMV Dose (Figure 4) (H&E 20×)
5. Discussion
Chromium is one of the most widely used industrial metals. Several million workers worldwide are estimated to be exposed to chromium compounds in an array of industries, such as pigment production, chrome plating, stainless steel welding, textile manufacturing, and leather tanning (1-3, 29, 30). A high incidence of lung injuries has been reported in workers exposed to certain inhaled particulate Cr(VI) compounds. These workers were found to have increased incidences of pulmonary congestion, edema, lung tissue fibrosis, and hyperplasia of the bronchial epithelium (1-4, 10). A large body of evidence supports the view that Cr(VI)-induced pulmonary toxicity in rats is similar to that reported in humans exposed to Cr(VI) (10, 13, 29, 30).
The results of the present study indicated that the level of GSH was significantly (P ≤ 0.05) decreased and the level of MDA was significantly increased in the lungs of rats treated with various doses of Cr(VI), compared to the control group. Soudani et al. (2013) reported that the lungs of rats exposed to Cr(VI) showed increased MDA and decreased GSH levels (11). Acharya et al. (2004) observed biochemical alterations in rat lung tissues after i.p. administration of Cr(VI) (31). Garcia-Nino et al. (2013) studied the effect of Cr(VI) on oxidative stress in rats. These authors reported that Cr(VI) enhanced the level of oxidative stress (32). The present study showed that Cr(VI) induced dose-dependent cell injury in the rat lung. Zhao et al. (2014) showed that intratracheal instillation of potassium dichromate at doses of 0.063 and 0.630 mg/kg for four weeks produced Clara cell injury in the rat lung (33). Fongmoon et al. (2014) showed that hexavalent chromium caused marked alveolar thickening associated with fibroblast and myofibroblast proliferation and collagen production in interstitial tissue, leading to pulmonary fibrosis in a rat model (34). An in vitro study indicated that Cr(VI) produced oxidative stress and induced apoptosis of lung epithelial cells (35). In the present study, severe degeneration was observed in rat lungs exposed to Cr(VI). It is probable that increased ROS production in the lung tissue may be responsible for this organ damage, as reflected by the changes in MDA levels. Another player in the induction of these changes is the depletion of thiol groups in the lung. Both of these effects, in concert, led to the development of Cr(VI)-induced lung injury.
Jabbari et al. (2011) reported that SIMV protected the rat kidney against gentamicin-induced nephrotoxicity (22). Fouad et al. (2013) reported that SIMV treatment ameliorates injury to rat testes induced by cadmium toxicity (36). Stoll et al. (2005) expressed that the antioxidant effects of statins likely contribute to their clinical efficacy in treating cardiovascular disease, as well as other chronic conditions associated with increased oxidative stress in humans (37). The results of the present study demonstrate that SIMV at a concentration of 20 mg/kg BW/day gave significant protection to the animals against the chromium-induced oxidative damage. Hadi et al. (2013) reported that SIMV ameliorates myocardial ischemia/reperfusion injury (38). Ferreira et al. (2014) found that treatment of mice with 20 mg/kg of SIMV improved the pulmonary damage caused by exposure to tobacco smoke when compared to control animals (26).
To the best of our knowledge, the effect of SIMV on Cr(VI)-induced lung biochemical and histopathological alterations had not been previously reported. Our findings are the first report of an in vivo study providing evidence for the beneficial effects SIMV on lungs exposed to Cr(VI).
In conclusion, the present study demonstrated that SIMV protected the lung against Cr(VI)-induced biochemical and histopathological changes in rats. The mechanisms of this pulmonary protective effect mainly include amelioration of lipid peroxidation induced by Cr(VI), as well as elevation of GSH levels.
Acknowledgements
References
-
1.
Scarselli A, Binazzi A, Marzio DD, Marinaccio A, Iavicoli S. Hexavalent chromium compounds in the workplace: assessing the extent and magnitude of occupational exposure in Italy. J Occup Environ Hyg. 2012;9(6):398-407. [PubMed ID: 22577838]. https://doi.org/10.1080/15459624.2012.682216.
-
2.
Caglieri A, Goldoni M, Acampa O, Andreoli R, Vettori MV, Corradi M, et al. The effect of inhaled chromium on different exhaled breath condensate biomarkers among chrome-plating workers. Environ Health Perspect. 2006;114(4):542-6. [PubMed ID: 16581543].
-
3.
Gatto NM, Kelsh MA, Mai DH, Suh M, Proctor DM. Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract: a meta-analysis. Cancer Epidemiol. 2010;34(4):388-99. [PubMed ID: 20430714]. https://doi.org/10.1016/j.canep.2010.03.013.
-
4.
Kerger BD, Paustenbach DJ, Corbett GE, Finley BL. Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicol Appl Pharmacol. 1996;141(1):145-58. [PubMed ID: 8917687]. https://doi.org/10.1006/taap.1996.0271.
-
5.
Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101(1):159-70. [PubMed ID: 17934191]. https://doi.org/10.1093/toxsci/kfm260.
-
6.
Seidler A, Jahnichen S, Hegewald J, Fishta A, Krug O, Ruter L, et al. Systematic review and quantification of respiratory cancer risk for occupational exposure to hexavalent chromium. Int Arch Occup Environ Health. 2013;86(8):943-55. [PubMed ID: 23079792]. https://doi.org/10.1007/s00420-012-0822-0.
-
7.
Li Chen T, LaCerte C, Wise SS, Holmes A, Martino J, Wise JJ, et al. Comparative cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human and sperm whale (Physeter macrocephalus) skin cells. Comp Biochem Physiol C Toxicol Pharmacol. 2012;155(1):143-50. [PubMed ID: 21466859]. https://doi.org/10.1016/j.cbpc.2011.03.011.
-
8.
Kumar D, Gangwar SP. Role of antioxidants in detoxification of Cr (VI) toxicity in laboratory rats. J Environ Sci Eng. 2012;54(3):441-6. [PubMed ID: 24749208].
-
9.
Xiao F, Li Y, Dai L, Deng Y, Zou Y, Li P, et al. Hexavalent chromium targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent caspase-3 activation in L-02 hepatocytes. Int J Mol Med. 2012;30(3):629-35. [PubMed ID: 22710416]. https://doi.org/10.3892/ijmm.2012.1031.
-
10.
Adams RJ, Wilson DH, Taylor AW, Daly A, Tursan d'Espaignet E, Dal Grande E, et al. Coexistent chronic conditions and asthma quality of life: a population-based study. Chest. 2006;129(2):285-91. [PubMed ID: 16478843]. https://doi.org/10.1378/chest.129.2.285.
-
11.
Soudani N, Rafrafi M, Ben Amara I, Hakim A, Troudi A, Zeghal KM, et al. Oxidative stress-related lung dysfunction by chromium(VI): alleviation by Citrus aurantium L. J Physiol Biochem. 2013;69(2):239-53. [PubMed ID: 22972417]. https://doi.org/10.1007/s13105-012-0207-6.
-
12.
Schneider BC, Constant SL, Patierno SR, Jurjus RA, Ceryak SM. Exposure to particulate hexavalent chromium exacerbates allergic asthma pathology. Toxicol Appl Pharmacol. 2012;259(1):38-44. [PubMed ID: 22178736]. https://doi.org/10.1016/j.taap.2011.12.001.
-
13.
Antonini JM, Roberts JR, Schwegler-Berry D, Mercer RR. Comparative microscopic study of human and rat lungs after overexposure to welding fume. Ann Occup Hyg. 2013;57(9):1167-79. [PubMed ID: 23798603]. https://doi.org/10.1093/annhyg/met032.
-
14.
Asatiani N, Kartvelishvili T, Abuladze M, Asanishvili L, Sapojnikova N. Chromium (VI) can activate and impair antioxidant defense system. Biol Trace Elem Res. 2011;142(3):388-97. [PubMed ID: 20809274]. https://doi.org/10.1007/s12011-010-8806-y.
-
15.
Samuel JB, Stanley JA, Vengatesh G, Princess RA, Muthusami S, Roopha DP, et al. Ameliorative effect of vitamin C on hexavalent chromium-induced delay in sexual maturation and oxidative stress in developing Wistar rat ovary and uterus. Toxicol Ind Health. 2012;28(8):720-33. [PubMed ID: 22033423]. https://doi.org/10.1177/0748233711422728.
-
16.
Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-88. [PubMed ID: 10079098]. https://doi.org/10.1172/JCI5909.
-
17.
Moon GJ, Kim SJ, Cho YH, Ryoo S, Bang OY. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J Clin Neurol. 2014;10(2):140-7. [PubMed ID: 24829600]. https://doi.org/10.3988/jcn.2014.10.2.140.
-
18.
Zeng WJ, Xiong CM, Zhao L, Shan GL, Liu ZH, Xue F, et al. Atorvastatin in pulmonary arterial hypertension (APATH) study. Eur Respir J. 2012;40(1):67-74. [PubMed ID: 22362846]. https://doi.org/10.1183/09031936.00149011.
-
19.
Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coronary Artery Dis. 2004;15(5):251-8. https://doi.org/10.1097/01.mca.0000131573.31966.34.
-
20.
Shyamsundar M, McKeown ST, O'Kane CM, Craig TR, Brown V, Thickett DR, et al. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179(12):1107-14. [PubMed ID: 19324974]. https://doi.org/10.1164/rccm.200810-1584OC.
-
21.
Dashti-Khavidaki S, Moghaddas A, Heydari B, Khalili H, Lessan-Pezeshki M, Lessan-Pezeshki M. Statins against drug-induced nephrotoxicity. J Pharm Pharm Sci. 2013;16(4):588-608. [PubMed ID: 24210066].
-
22.
Jabbari M, Rostami Z, Jenabi A, Bahrami A, Mooraki A. Simvastatin ameliorates gentamicin-induced renal injury in rats. Saudi J Kidney Dis Transpl. 2011;22(6):1181-6. [PubMed ID: 22089778].
-
23.
Buege JA, Aust SD. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10. https://doi.org/10.1016/s0076-6879(78)52032-6.
-
24.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-7. https://doi.org/10.1016/0003-9861(59)90090-6.
-
25.
Goodarzi Z, Karami E, Ahmadizadeh M, Ahmadi Angali K. The Effect of Simvastatin on Sodium Dichromate-Induced Oxidative Stress. Jundishapur J Health Sci. 2014;6(4). https://doi.org/10.5812/jjhs.23463.
-
26.
Ferreira TS, Lanzetti M, Barroso MV, Rueff-Barroso CR, Benjamim CF, de Brito-Gitirana L, et al. Oxidative stress and inflammation are differentially affected by atorvastatin, pravastatin, rosuvastatin, and simvastatin on lungs from mice exposed to cigarette smoke. Inflammation. 2014;37(5):1355-65. [PubMed ID: 24609836]. https://doi.org/10.1007/s10753-014-9860-y.
-
27.
Ozbakis-Dengiz G, Hekimoglu A, Kandemir N, Kurcer Z. Effects of statins in an indomethacin-induced gastric injury model in rats. Turk J Gastroenterol. 2012;23(5):456-62. [PubMed ID: 23161290].
-
28.
Mohammadi S, Zamani E, Mohadeth Z, Mogtahedi F, Chopan H, Moghimi F, et al. Effects of Different Doses of Simvastatin on Lead-Induced Kidney Damage in Balb/C Male Mice. Pharm Sci. 2015;20:157.
-
29.
Elhosary N, Maklad A, Soliman E, El-Ashmawy N, Oreby M. Evaluation of oxidative stress and DNA damage in cement and tannery workers in Egypt. Inhal Toxicol. 2014;26(5):289-98. [PubMed ID: 24617565]. https://doi.org/10.3109/08958378.2014.885100.
-
30.
Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS. Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal. 2004;24(5):1099-108. [PubMed ID: 15563281]. https://doi.org/10.1111/j.0272-4332.2004.00512.x.
-
31.
Acharya UR, Mishra M, Mishra I. Status of antioxidant defense system in chromium-induced Swiss mice tissues. Environ Toxicol Pharmacol. 2004;17(3):117-23. [PubMed ID: 21782722]. https://doi.org/10.1016/j.etap.2004.02.005.
-
32.
Garcia-Nino WR, Tapia E, Zazueta C, Zatarain-Barron ZL, Hernandez-Pando R, Vega-Garcia CC, et al. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid Based Complement Alternat Med. 2013;2013:424692. [PubMed ID: 23956771]. https://doi.org/10.1155/2013/424692.
-
33.
Zhao L, Song Y, Pu J, Guo J, Wang Y, Chen Z, et al. Effects of repeated Cr(VI) intratracheal instillation on club (Clara) cells and activation of nuclear factor-kappa B pathway via oxidative stress. Toxicol Lett. 2014;231(1):72-81. [PubMed ID: 25240275]. https://doi.org/10.1016/j.toxlet.2014.09.011.
-
34.
Fongmoon D, Pongnikorn S, Chaisena A, Iamsaard S. Particulate matters collected from ceramic factories in Lampang Province affecting rat lungs. J Zhejiang Univ Sci B. 2014;15(1):75-83. [PubMed ID: 24390747]. https://doi.org/10.1631/jzus.B1300058.
-
35.
Lei T, He QY, Cai Z, Zhou Y, Wang YL, Si LS, et al. Proteomic analysis of chromium cytotoxicity in cultured rat lung epithelial cells. Proteomics. 2008;8(12):2420-9. [PubMed ID: 18563748]. https://doi.org/10.1002/pmic.200701050.
-
36.
Fouad AA, Albuali WH, Jresat I. Simvastatin treatment ameliorates injury of rat testes induced by cadmium toxicity. Biol Trace Elem Res. 2013;153(1-3):269-78. [PubMed ID: 23625729]. https://doi.org/10.1007/s12011-013-9667-y.
-
37.
Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Timely Top Med Cardiovasc Dis. 2005;9:E1. [PubMed ID: 15824760].
-
38.
Hadi NR, Al-Amran F, Yousif M, Zamil ST. Antiapoptotic effect of simvastatin ameliorates myocardial ischemia/reperfusion injury. ISRN Pharmacol. 2013;2013:815094. [PubMed ID: 24455299]. https://doi.org/10.1155/2013/815094.