Abstract
Background:
Hypertension is one of the major risk factors for cardiovascular diseases. Lifestyle changes, physical exercise and intake of healthy diet are some common issues associated with reducing the risk of hypertension. There are several classes of pharmacological agents used in the treatment of hypertension. Angiotensin I converting enzyme (ACE) cleaves angiotensin I to angiotensin II and inactivates bradykinin. The synthetic ACE inhibitors are used widely to treat cardiovascular disorders. They may cause many adverse effects, including dry cough, allergic reactions and skin rashes, so investigation for new natural sources could be helpful.Objectives:
The aim of the present study was to evaluate angiotensin I converting enzyme inhibitory activities of hydroalcoholic extracts of Nardostachys jatamansi, Prangos ferulacea and Marrubium vulgare.Materials and Methods:
ACE inhibitory activity was measured according to the methods of Cushman & Cheung with some modifications. Captopril was used as positive control.Results:
The ACE inhibitory activities of hydroalcoholic extracts were as follows; M. vulgare > N. Jatamansi > P. ferulacea. The least IC50 value was related to the hydroalcoholic extract of M. vulgare (0.791 mg/mL). The IC50 values of N. Jatamansi and P. ferulacea were 2.147 and 4.057 mg/mL, respectively.Conclusions:
The results supported the traditional antihypertensive use of these plants, especially M. vulgare, by inhibitory effects on ACE enzyme.Keywords
Angiotensin-Converting Enzyme Inhibitors Hypertension Nardostachys jatamansi Marrubium vulgare Prangos ferulacea
1. Background
Hypertension is an important public health challenge because of its high prevalence. Its prevalence is estimated to be 29% until 2025 (1). High blood pressure is a main risk factor for cardiovascular disease. It is known as a major cause of coronary heart disease, cerebrovascular diseases, peripheral vascular disease and heart failure (2).
There are different drug classes for hypertension treatment. The inhibitors of angiotensin-converting enzyme (ACE) are one of the most important ones. ACE is a key component in the renin angiotensin aldosterone system (RAAS), which regulates blood pressure (3). However, most patients require two or more drugs to control high blood pressure (4).
ACE is a Nardostachys jatamansi with a zinc ion. The enzyme has a less substrate specificity in vitro. ACE consists of a single polypeptide chain containing two domains of N and C. There are two catalytic sites in each of these domains. ACE plays an important role in the regulation of blood pressure, by acting on the renin–angiotensin system (RAS) and the kallikrein-kinin system (KKS) (5). ACE converts angiotensin I to angiotensin II, which is a potent vasoconstrictor and stimulates adrenal cortex to secret aldosterone, leading to retention of sodium ions. In addition, ACE is responsible for deactivation of bradykinin as a vasodilator substance. Because of the dual effects of ACE in the maintenance of blood pressure and fluid and electrolyte homeostasis, inhibition of ACE has been verified in the treatment of hypertension and congestive heart failure status (6). Synthetic ACE inhibitors such as captopril and enalapril have some adverse effects such as cough, allergic reactions, taste disorders and skin rashes (7, 8).
There are incentives for further researches to find cheaper and more effective drugs, including plants. Currently, natural products and their derivatives include more than 50% of all medicines in clinical use (9). The plants have formed the basis of the traditional medicine introduced from thousands of years ago. Even in modern times, plant-based medications continue to play a major role in health care systems. The World Health Organization estimates that approximately 80% of population in developing countries use the traditional medicine for primary health care. The plant products also play an important role in health care for the remaining 20% (10).
There are many medicinally antihypertensive herbs such as Lime blossom (Tilia europea), Kudzu (Pueraria lobata), Garlic (Allium sativum), Saffron (Crocus sativus), Valerian (Valeriana officinalis), Mistletoe (Viscum album) and Rauwolfia serpentine. They are devoid of side effects like weakness, tiredness, drowsiness, impotence, cold hands and feet, depression, insomnia, abnormal heartbeats, skin rash, dry mouth, dry cough, headache, dizziness, constipation or diarrhea and do not have any interaction with other antihypertensive drugs (11).
The plants in this study were Nardostachys jatamansi (Indian Valerian), Prangos ferulacea and Marrubium vulgare (Horehound). They have been used for hypertension treatment in the traditional medicine (12).
2. Objectives
The aim of the present study was to evaluate angiotensin I converting enzyme inhibitory activities of three plants mentioned above.
3. Materials and Methods
3.1. Chemicals
All the chemicals were purchased from sigma-Aldrich Chemie Gmbh (USA) and Merck (Germany) companies. The chemicals were of analytical grade. ACE (angiotensin-converting enzyme, EC 3.4.15.1) from rabbit lung, substrate (HHL, hippuryl-histidyl-leucine) of ACE (St. Louis, MO) and all other chemicals were used reagent grade chemicals.
3.2. Plant Materials
The rhizome of N. jatamansi and the flowering branches of P. ferulacea were collected in Khuzestan and aerial parts of M. vulgar were collected in Mazandaran province during the flowering period in summer 2010. The plants were identified at the Herbarium of Department of Pharmacognosy, school of pharmacy, Ahvaz, Iran, where voucher specimens were preserved.
3.3. Extraction
The plant material (200 g) was powdered and extracted with EtOH-water (80 - 20) using maceration method. Then the extract was filtered and concentrated under reduced pressure with rotary evaporator (Heidolf, Germany). Next, lyophilized by freeze dryer (Operon, Korea). The obtained powders were kept in amber glass containers in a refrigerator.
3.4. ACE Inhibition Assay
ACE hydrolyses angiotensin I analog (HHL) and ACE activity is determined according to the amount of produced hippuric acid as follows:
Hippuryl-L-Histidyl-L-Leucine → Hippuric acid + L-Histidyl-L-Leucine
The ACE inhibitory activity was measured by the method of Cushman and Cheung method (1971) with slight modifications. Fifty microliters of the inhibitor (different concentrations of extracts and positive control) with 50 μL of ACE (25 unit/mL) was incubated at 37°C for 10 minutes, then 150 μL of substrate (8.3 mM HHL in 50 mM sodium borate buffer containing 0.5 M NaCl at pH 8.3) was added and incubated again at 37°C for 30 minutes. The reaction was terminated by adding 250 μL of 1.0 M HCl. The produced hippuric acid was extracted with 500 μL of ethylacetate. After centrifugation (800 g, 15 minutes) 200 μL of the upper layer was transferred into a test tube and evaporated at room temperature for two hours in a vacuum. Hippuric acid was dissolved in 1.0 mL of distilled water. Finally, the absorbance was measured at 228 nm using a UV-spectrophotometer (Jasco, Japan) (13).
ACE inhibition test was performed on three samples: positive control, negative control and fractions prepared from three mentioned plants. Captopril was used as positive control.
The following concentrations of 0.0078, 0.0156, 0.0312, 0.0625, 0.125, 0.25 and 0.5 mg/mL of captopril were used. Following extract concentrations were prepared 0.625, 1.25, 2.5, 3.75 and 5 mg/mL by serial dilution method. For blank preparation, borate buffer was used instead of enzyme solution. The negative control had no ACE inhibitors and maximum absorbance was related to negative control.
3.5. Statistical Analysis
For all tests, the inhibition assay was performed in triplicate and ACE inhibition percentage calculated as follows:
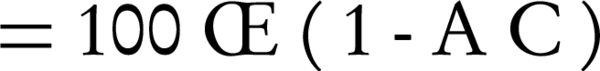
A = Absorbance test - Absorbance blank
C = Absorbance negative control
Linear chart was plotted for ACE inhibition percentage versus the logarithm of concentration for each sample. The IC50 values were determined by constructing a dose-inhibition curve. The IC50 value was defined as the concentration of inhibitor required to inhibit 50% of the ACE. IC50 values can be used to compare the potency of plants extract.
4. Results
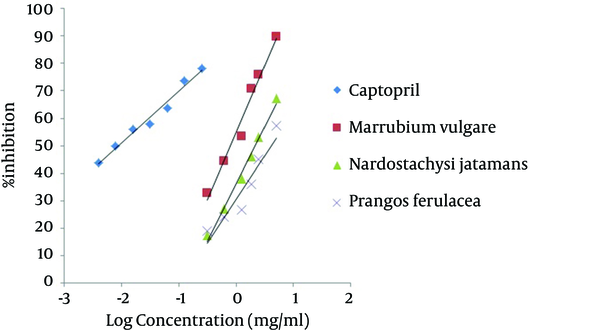
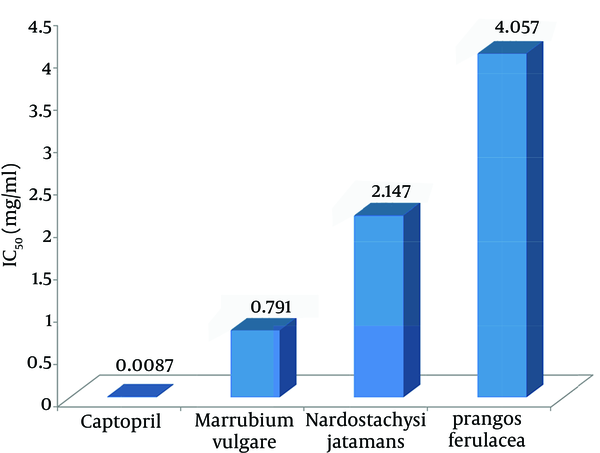
Plants extracted with Ethanol-water (80 - 20) using maceration method and the percentage yield of plant extract entered in Table 1. We evaluated the inhibitory effects of hydroalcoholic extracts of N. jatamansi, P. ferulacea and M. vulgare on ACE enzyme. The ACE inhibitory activities of plants were represented as percentage ACE inhibition by the extracts. Percentage of ACE inhibition by different concentrations of captopril and studied plants was calculated. The plants demonstrated ACE inhibitory activities in a concentration dependent manner (Figure 1). The IC50 values of hydroalcoholic extract of M. vulgare, N. jatamansi, and P. ferulacea were 0.791, 2.147 and 4.057 mg/mL, respectively and that of standard, captopril, was 0.0087 mg/mL (Figure 2).
| Plants | Yield |
|---|---|
| Marrubium vulgare | 8.63 |
| Nardostachys jatamansi | 6.74 |
| Prangos ferulacea | 11.32 |
ACE Inhibition Percentage of Different Concentrations of N. jatamansi, P. ferulacea, M. vulgare and Captopril
IC50 value of Nardostachys jatamansi, Prangos ferulacea and Marrubium vulgare Compared to Captopril (mg/mL)
5. Discussion
This study showed that M. vulgare has the least IC50 (0.791 mg/mL) and the most inhibitory effect among the studied plants. The IC50 of Indian valerian was 2.147 mg/mL, which shows that the inhibitory effect is less than M. vulgare and more than P. ferulacea. P. ferulacea had the minimal inhibitory effect among the other plants with IC50 = 4.057 mg/mL. Previous studies showed antioxidant (14), antibacterial (15) and abortifacient effects of P. ferulacea (16, 17).
In a study, nine herbs used in the traditional medicine of Lebanon were tested about their inhibitory effect on ACE enzyme. N- hexane extract of Hyssopus officinalis had the least IC50 and so the best inhibitory effect among them with IC50 = 52 µg/mL. According to our results, its inhibitory effect is more than our studied plants. Chloroform extract of M. radiatum had IC50 of 110 µg/mL, but the IC50 of hydroalcoholic and n-hexane extract was between 60 - 65 µg/mL. Hydroalcoholic extract of M. vulgare in our study had a less inhibitory effect than hydroalcoholic, n-hexane and chloroform extract of M. radiatum (12).
Previous studies showed analgesic (18), antispasmodic (19), anti-diabetic (20), hepatoprotective (21, 22), antioxidant effects and (23, 24) prevention of hyper-cholesterolemia (25) of M. vulgare.
In a study, 20 traditional herbs of Zulu were selected for screening of ACE inhibitory effect and the most inhibitory effect related to Adenopodia spicata (26).
Antioxidant effect of M. vulgare has been proven due to having flavonoid and phenolic acid. This plant contains tannin and the inhibitory effect of tannins and flavonoid on ACE has been demonstrated (27). In our study it had the least IC50.
Another checking plant is N. jatamansi, which its effects on improving memory and learning (28), treatment of nervous headache (29) and hypertension (30) have been proven.
In a study on aqueous extract of Salviae miltiorrhiza root in Korea for assessing ACE inhibitory effect, obtained IC50 was 170 µg/mL. This plant is a rich source of pigments and some phenolic compounds like lithospermic acid B (31).
In another study on leaves of Vaccinium ashei reade, obtained IC50 was 46 µg/mL. Inhibitory effect is more than the three plants in our study. This plant contains 18.7% tannin. Perhaps, this proper inhibitory effect is related to tannins percentage in this plant. Tannins are polyphenolic compounds that their inhibitory effect on ACE has been proven (32).
Muthuswamy Umamaheswari in India performed a study on seed extract of Apium graveolens Linn. (celery) and observed potential antioxidant and ACE inhibitory effects (IC50 = 666.26 μg/mL). Phytochemical screening of the extract showed that it contains tannins, phenolics, flavonoids glycosides and steroids (33).
Phoenix sylvestris is used as a diuretic in India. A study found that this plant has 48% inhibitory effect in 0.33 mg/mL concentration. Moreover, if we remove tannins of this plant, its inhibitory effect reduces to 8%, indicating the inhibitory role of tannins (34).
Due to non-specific ACE inhibitors and adverse effects of chemical inhibitors of ACE, it is necessary to investigate for new natural compounds.
The present study was conducted to assess anti-hypertensive effects according to ACE inhibition activity of three different spices used as traditional medicinal herbs in Iran. It is suggested to perform further clinical studies to confirm the effects and safety of the studied plants.
Acknowledgements
References
-
1.
Tousoulis D, Androulakis E, Papageorgiou N, Stefanadis C. Novel therapeutic strategies in the management of arterial hypertension. Pharmacol Ther. 2012;135(2):168-75. [PubMed ID: 22609833]. https://doi.org/10.1016/j.pharmthera.2012.05.004.
-
2.
Kramoh EK, N'Goran Y N, Ake-Traboulsi E, Anzouan-Kacou JB, Konin CK, Coulibaly I, et al. Hypertension management in an outpatient clinic at the Institute of Cardiology of Abidjan (Ivory Coast). Arch Cardiovasc Dis. 2011;104(11):558-64. [PubMed ID: 22117907]. https://doi.org/10.1016/j.acvd.2011.08.002.
-
3.
Wilson J, Hayes M, Carney B. Angiotensin-I-converting enzyme and prolyl endopeptidase inhibitory peptides from natural sources with a focus on marine processing by-products. Food Chem. 2011;129(2):235-44. https://doi.org/10.1016/j.foodchem.2011.04.081.
-
4.
Oliveira-Paula GH, Lacchini R, Coeli-Lacchini FB, Junior HM, Tanus-Santos JE. Inducible nitric oxide synthase haplotype associated with hypertension and responsiveness to antihypertensive drug therapy. Gene. 2013;515(2):391-5. [PubMed ID: 23266817]. https://doi.org/10.1016/j.gene.2012.12.059.
-
5.
Ko SC, Kang N, Kim EA, Kang MC, Lee SH, Kang SM, et al. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012;47(12):2005-11. https://doi.org/10.1016/j.procbio.2012.07.015.
-
6.
Javad Mousavi SA, Yadollah Zadeh M, Hossein Nejad Yazdi M, Adeli SH. Spirometric Assessment of Patients Having Opium Smoking Addiction and Pulmonary Complications. Razi J Med Sci. 2005;12(46):267-74.
-
7.
Hajji M, Masmoudi O, Souissi N, Triki Y, Kammoun S, Nasri M. Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chem. 2010;121(3):724-31. https://doi.org/10.1016/j.foodchem.2010.01.021.
-
8.
Lahogue V, Réhel K, Taupin L, Haras D, Allaume P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010;118(3):870-5. https://doi.org/10.1016/j.foodchem.2009.05.080.
-
9.
Raji IA, Mugabo P, Obikeze K. Effect of Tulbaghia violacea on the blood pressure and heart rate in male spontaneously hypertensive Wistar rats. J Ethnopharmacol. 2012;140(1):98-106. [PubMed ID: 22222281]. https://doi.org/10.1016/j.jep.2011.12.034.
-
10.
Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101(12):4676-89. [PubMed ID: 20185303]. https://doi.org/10.1016/j.biortech.2010.01.093.
-
11.
Jha AK, Jha M, Kaur J. Research Journal of Pharmaceutical, Biological and Chemical Sciences.
-
12.
Loizzo MR, Saab AM, Tundis R, Menichini F, Bonesi M, Piccolo V, et al. In vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. J Ethnopharmacol. 2008;119(1):109-16. [PubMed ID: 18601990]. https://doi.org/10.1016/j.jep.2008.06.003.
-
13.
Je JY, Park PJ, Kim EK, Ahn CB. Antioxidant and angiotensin I converting enzyme inhibitory activity of Bambusae caulis in Liquamen. Food Chem. 2009;113(4):932-5. https://doi.org/10.1016/j.foodchem.2008.08.022.
-
14.
Coruh N, Celep A, Ozgokce F. Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2007;100(3):1237-42. https://doi.org/10.1016/j.foodchem.2005.12.006.
-
15.
Durmaz H, Sagun E, Tarakci Z, Ozgokce F. Antibacterial activities of Allium vineale, Chaerophyllum macropodum and Prangos ferulacea. Afr J Biotechnol. 2006;5(19).
-
16.
Kazerooni T, Mousavizadeh K, Abdollahee A, Sarkarian M, Sattar A. Abortifacient effect of Prangos ferulacia on pregnant rats. Contraception. 2006;73(5):554-6. [PubMed ID: 16627045]. https://doi.org/10.1016/j.contraception.2005.11.001.
-
17.
Kazerooni T, Mousavizadeh K. Effect of Prangos Ferulacea on abortion on pregnant rats. Fertil Steril. 2005;84:S168-9.
-
18.
de Souza MM, de Jesus RA, Cechinel-Filho V, Schlemper V. Analgesic profile of hydroalcoholic extract obtained from Marrubium vulgare. Phytomedicine. 1998;5(2):103-7. [PubMed ID: 23195761]. https://doi.org/10.1016/S0944-7113(98)80005-6.
-
19.
Schlemper V, Ribas A, Nicolau M, Cechinel Filho V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine. 1996;3(2):211-6. [PubMed ID: 23194972]. https://doi.org/10.1016/S0944-7113(96)80038-9.
-
20.
Boudjelal A, Henchiri C, Siracusa L, Sari M, Ruberto G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia. 2012;83(2):286-92. [PubMed ID: 22100836]. https://doi.org/10.1016/j.fitote.2011.11.005.
-
21.
Verma A, Masoodi M, Ahmed B. Lead finding from whole plant of Marrubium vulgare L. with Hepatoprotective Potentials through in silico methods. Asian Pac J Trop Biomed. 2012;2(3):S1308-11. https://doi.org/10.1016/S2221-1691(12)60406-7.
-
22.
Akther N, Shawl AS, Sultana S, Chandan BK, Akhter M. Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J Pharm Res. 2013;7(7):565-70. https://doi.org/10.1016/j.jopr.2013.06.023.
-
23.
Pukalskas A, Venskutonis PR, Salido S, de Waard P, van Beek TA. Isolation, identification and activity of natural antioxidants from horehound (Marrubium vulgare L.) cultivated in Lithuania. Food Chem. 2012;130(3):695-701. https://doi.org/10.1016/j.foodchem.2011.07.112.
-
24.
Orhan IE, Belhattab R, Şenol FS, Gülpinar AR, Hoşbaş S, Kartal M. Profiling of cholinesterase inhibitory and antioxidant activities of Artemisia absinthium, A. herba-alba, A. fragrans, Marrubium vulgare, M. astranicum, Origanum vulgare subsp. glandulossum and essential oil analysis of two Artemisia species. Ind Crops Prod. 2010;32(3):566-71. https://doi.org/10.1016/j.indcrop.2010.07.005.
-
25.
Roughani M. Analgesic Effect of Chronic Oral Administration of Aerial Part of Marrubium Vulgare in Diabetic Rats. J Babol Univ Med Sci. 2006;8(2):7-13.
-
26.
Duncan AC, Jager AK, van Staden J. Screening of Zulu medicinal plants for angiotensin converting enzyme (ACE) inhibitors. J Ethnopharmacol. 1999;68(1-3):63-70. [PubMed ID: 10624863].
-
27.
Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94(4):550-7. https://doi.org/10.1016/j.foodchem.2004.12.004.
-
28.
Joshi H, Parle M. Nardostachys jatamansi improves learning and memory in mice. J Med Food. 2006;9(1):113-8. [PubMed ID: 16579738]. https://doi.org/10.1089/jmf.2006.9.113.
-
29.
Chauhan R, Nautiyal M. Commercial viability of cultivation of an endangered medicinal herb Nardostachys jatamansi at three different agroclimatic zones. Curr Sci Bangalore. 2005;89(9):1481.
-
30.
Rucker G, Tautges J, Sieck A, Wenzl H, Graf E. [Isolation and pharmacodynamic activity of the sesquiterpene valeranone from Nardostachys jatamansi DC]. Arzneimittelforschung. 1978;28(1):7-13. [PubMed ID: 580202].
-
31.
Kang DG, Yun YG, Ryoo JH, Lee HS. Anti-hypertensive effect of water extract of danshen on renovascular hypertension through inhibition of the renin angiotensin system. Am J Chin Med. 2002;30(1):87-93. [PubMed ID: 12067101]. https://doi.org/10.1142/S0192415X02000107.
-
32.
Sakaida H, Nagao K, Higa K, Shirouchi B, Inoue N, Hidaka F, et al. Effect of Vaccinium ashei reade leaves on angiotensin converting enzyme activity in vitro and on systolic blood pressure of spontaneously hypertensive rats in vivo. Biosci Biotechnol Biochem. 2007;71(9):2335-7. [PubMed ID: 17827680]. https://doi.org/10.1271/bbb.70277.
-
33.
Umamaheswari M, Ajith MP, Asokkumar K, Sivashanmugam T, Subhadradevi V, Jagannath P. In vitro angiotensin converting enzyme inhibitory and antioxidant activities of seed extract of Apium graveolens Linn. Ann Biol Res. 2012;3(3):1274-82.
-
34.
Braga FC, Serra CP, Viana NJ, Oliveira AB, Cortes SF, Lombardi JA. Angiotensin-converting enzyme inhibition by Brazilian plants. Fitoterapia. 2007;78(5):353-8. [PubMed ID: 17513067]. https://doi.org/10.1016/j.fitote.2007.02.007.