Abstract
Keywords
Phenolics Flavonoid Antioxidant Antimicrobial Activity Nepeta binaludensis
1. Background
The genus Nepeta (Lamiaceae) includes about 250 species, which are found in North Africa, Eurasia, particularly the Mediterranean region. There are about 75 species grown wild in Iran, of which 46 species are endemic (1). Nepeta binaludensis Jamzad is a rare and endemic aromatic plant grown in limited areas of Northeast Iran in the Binalood Mountains (2). The plant is widely used in traditional medicine for headaches, migraines, digestive disorders, rheumatism, asthma, and cardiovascular disorders (3).
Based on the authors’ previous studies on Iranian medicinal and aromatic plants, various extracts of Nepeta binaludensis were investigated in terms of total phenolic and flavonoid contents as well as antimicrobial and antioxidant activities. There are a few studies on N. binaludensis, but not in line with the current study. Rustaiyan et al. reported that the essential oil of N. binaludensis is rich in 1,8-cineole (4). Mohammadpour et al. reported significant antibacterial activity for the essential oil of the species (5). There are also two reports by Nadjafi et al. on N. binaludensis. In the first study, essential oil composition of the plant was evaluated and 1,8-cineole was reported as the main component; they also studied the eco-biological background and ethno-medicinal use of the rare and highly endangered species, N. binaludensis (3). In the second study, the effects of cultivation on phenology and morphological properties of the plant were investigated (6).
2. Methods
2.1. Chemicals and Instrumental
Aluminum chloride, Folin-Ciocalteu reagent, quercetin, sodium acetate, sodium bicarbonate, butylated hydroxyltoluene (BHT), and the other solvents were prepared from Merck (Germany). Gallic acid and 1,1-diphenyl-2-picrylhedrazyl (DPPH) were purchased from Sigma-Aldrich (US). For colorimetric methods and DPPH assay, UV-visible spectrophotometer (CECIL, CE 7800, UK) was used. MHA (Muller-Hinton agar), MHB (Muller-Hinton broth) and BHIA (brain-heart infusion agar) were purchased from Merck (Germany) and all the antibiotics and antifungal agents were provided from Sigma-Aldrich (US).
2.2. Plant Material
The aerial parts of Nepeta binaludensis Jamzad were collected from Zoshk Village region in Binalood Mountains (3000 m height), in Khorasan Province, Iran on June 2014. Voucher specimen (No.48860) was deposited at the Herbarium of the Research Institute of Forests and Rangelands (TARI), Tehran, Iran.
2.3. Preparation of the Extracts
To prepare the extract, 10 g of dried powdered aerial parts of N. binaludensis was mixed with 100 mL of the following solvents separately: methanol, 50% hydro-methanol, deionized water, and ethyl acetate. The mixture was stirred occasionally in one week and then, was passed through a filter paper. The filtrate was concentrated under reduced pressure and used for assays.
2.4. Total Phenolic Contents
Total phenolic contents of the extracts were determined by the Folin-Ciocalteu method (7). Briefly, 0.75 mL of Folin-Ciocalteu reagent (10-fold diluted by deionized water) was added to 100 μL of the extracts (methanol, 50% hydro-methanol, and distilled water) (20 mg/mL) at room temperature. After 5 minutes, 0.75 mL of sodium bicarbonate (60 g/L) was added and mixed thoroughly.
The absorbance rate of each extract was measured by UV-Vis spectrophotometer after 90 minutes.
A calibration curve was plotted for the standard solutions of gallic acid (0 - 100 ppm) and total phenolic contents of the extracts were evaluated in terms of gallic acid equivalent (mg/L).
The results were expressed as mean ± standard deviation (SD) for three independent experiments.
2.5. Total Flavonoid Contents
Aluminum chloride colorimetric method was used to estimate total flavonoid contents of the extracts (8).
Briefly, 0.5 mL of each extract (methanol, 50% hydro-methanol, and deionized water) (20 mg/mL) was dissolved in methanol (1.5 mL) and then, 10% aluminum chloride (0.1 mL), deionized water (2.8 mL), and 1.0 M sodium acetate (0.1 mL) were added. The solutions were incubated for 30 minutes at room temperature. Then, the absorbance of the solutions was measured at 415 nm by UV-Visible spectrophotometer. The same procedure was used for the standard solutions of quercetin (0 - 100 ppm) and a calibration curve was plotted. The flavonoid contents of the extracts were expressed in terms of quercetin equivalent (mg/L). The results were expressed as mean ± SD for three independent experiments.
2.6. Antioxidant Activity
Radical scavenging activity of the extracts and the standard (BHT) was measured from the bleaching of the purple-colored methanolic solution of DPPH (9). For this purpose, a solution of DPPH in methanol (40 μg/mL) was freshly prepared. Then, 2.5 µL of the solution was added to 10μL of each extract. Finally, the absorbance of the test tubes was measured by the spectrophotometer at 517 nm after 30 minutes incubation in dark. To calculate the percentage of radical scavenging activity, following equation was employed:
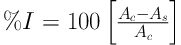
Where As is the absorbance of sample and Ac is the absorbance of control (containing all reagents except the test sample).
The average of two independent experiments was expressed as the final result.
The IC50 value (extract concentration providing 50% inhibition) was calculated by the graph plotting inhibition percentage against the extracts concentration.
2.7. Antimicrobial Activity
Crude extracts of N. binaludensis was used to evaluate antimicrobial activity based on the macrodilution method. In this method, the ability of microorganisms to produce visible growth in the tubes containing serial dilutions of the antimicrobial agents is tested. For this purpose, 50% hydro-methanol (117 mg/mL) and ethyl acetate (64 mg/mL) extracts of the plant were used. The microorganisms evaluated in the study were Streptococcus pyogenes (ATCC 8668), Staphylococcus aureus (ATCC 25923), Salmonella paratyphi B (ATCC 1231), Escherichia coli (ATCC 11229), and a fungus Candida albicans (ATCC 10231) prepared from research center of biotechnology and industrial center of fungi and bacteria collections, Iran. The microorganisms were maintained in viable state via incubation on MHA and overnight incubation at 37°C. Broth inoculums were standardized to 0.5 McFarland by mean of dilution with sterile MHB and measuring the optical density at 620 nm wavelength. Absorbance readings were set to 0.09 - 0.13 (~ 1.5 × 108 CFU/mL).
The standard antibiotics, including tetracycline, ampicillin, kanamycin (100 mg/mL), and an antifungal agent as nystatin (2.5 mg/mL), were used as positive controls. To determine the possible inhibitory activity of solvents on the tested bacteria, 50% hydro-methanol and ethyl acetate were used simultaneously as positive controls.
A twofold serial dilution of each extract was prepared in 12 tubes containing 1 mL Muller-Hinton broth; then, 0.1 mL of bacterial suspension with 0.5 McFarland turbidity was added to all the tubes, except the tube No. 12 (as the control). Then, the tubes were incubated at 37°C for 24 hours. To determine the MBC (minimum bacterial concentration, the lowest concentration of an antibacterial agent required to kill a particular bacterium), 25 μL of each dilution was incubated at 37°C in BHIA for 24 hours. The highest dilution (least concentration) that inhibited colony formation on this solid medium was considered as MBC (10).
3. Results and Discussion
The extracts obtained by the solvents were methanol, 50% hydro-methanol, deionized water, and ethyl acetate with 6.5%, 7.9%, 4.8%, 4.3% w/w, respectively. Methanol, 50% methanol, and aqueous extracts were used for the evaluation of total phenolic and flavonoids contents and DPPH scavenging assay. The ethyl acetate and 50% methanolic extracts were used for antimicrobial tests.
The way to determine the level of total phenolic compounds is based on their chemical reducing capacity relative to gallic acid. It was measured by Folin-Ciocalteu reagent in terms of gallic acid equivalent (standard curve equation: Y = 0.0105X + 0.0138, R2= 0.9955). As shown in Table 1, total phenolic contents in 50% hydro-methanol extract (114.97 ± 0.0017 mg/L, 4.42 ± 0.0014 mg/g) was higher than those of aqueous (41.23 ± 0.0025 mg/L, 2.29 ± 0.002 mg/g) and methanolic (38.11 ± 0.001 mg/L, 1.30 ± 0.011 mg/g) extracts.
| 50% Hydro-Methanol | Distilled Water | Methanol | |
|---|---|---|---|
| Absorbance at 765 nm | 1.22 ± 0.0025 | 0.571 ± 0.0034 | 0.414 ± 0.0011 |
| Phenolic content, mg/Lb | 114.97 ± 0.0017 | 41.23 ± 0.0025 | 38.11 ± 0.001 |
| Phenolic content, mg/gc | 4.42 ± 0.0014 | 2.29 ± 0.002 | 1.30 ± 0.011 |
| Absorbance at 415 nm | 0.794 ± 0.019 | 0.3 ± 0.0028 | 0.49 ± 0.004 |
| Flavonoid content, mg/Ld | 11.04 ± 0.013 | 4.39 ± 0.002 | 6.76 ± 0.0015 |
| Flavonoid content, mg/ge | 0.8 ± 0.0009 | 0.11 ± 0.0012 | 0.16 ± 0.0021 |
The flavonoid contents in terms of quercetin equivalent (the standard curve equation: Y = 0.0673X + 0.0051, R2 = 0.9961) showed that total flavonoid contents of 50% hydro-methanol extract (11.04 ± 0.013 mg/L, 0.8 ± 0.0009 mg/g) was higher than those of methanolic (6.76 ± 0.0015 mg/L, 0.16 ± 0.0021 mg/g) and aqueous (4.39 ± 0.002 mg/L, 0.11 ± 0.0012 mg/g) extracts (Table 1).
Possible antioxidant activity of the extracts was also investigated by DPPH scavenging assay in this project. Results showed the higher potency for the 50% hydro-methanol extract than the methanolic and aqueous extracts. The highest antioxidant activity was less than half-maximal inhibitory concentration (IC50) value. The IC50 value for 50% hydro-methanol, methanolic, and aqueous extracts were 3.0, 4.5, and 5.2 mg/mL, respectively (Figure 1). The IC50 value for BHT as a positive control was 8.1 mg/mL.
Radical scavenging effect of Nepeta binaludensis extracts in comparison with BHT
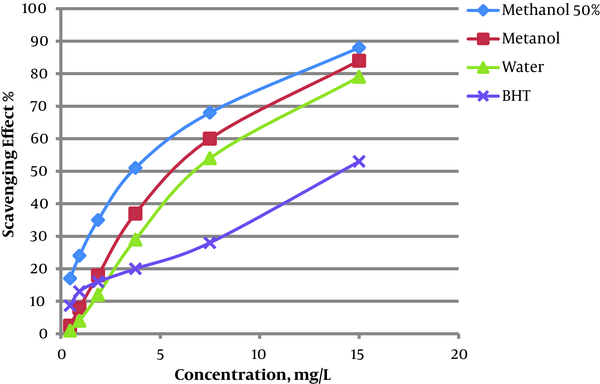
Based on the obtained results, there was a good correlation between antioxidant activity and total flavonoid and phenolic contents in the various extracts of N. binaludensis. Phenolic compounds and flavonoids are the secondary metabolites with powerful radical scavenging effect and antioxidant activity (11).
Because of high amounts of flavonoids and phenolic compounds in 50% hydro-methanol extract of N. binaludensis, the study evaluated antimicrobial activity of the mentioned extracts against a number of microorganisms to find a relationship between antimicrobial activity and phenolic and flavonoid contents of the extracts. Antimicrobial activity of the ethyl acetate extract was also evaluated for comparison. This solvent is a good choice to extract terpenoides, a class of phytochemicals, which is considered as an antimicrobial agent. Some antifungal and antibiotics were also used as positive controls simultaneously. The results of antimicrobial activity are given in Table 2 and Figure 2.
MBC of Nepeta binaludensis Various Extracts Against Several Microorganisms
Antimicrobial activity of 50% hydro-methanol (A - E) and ethyl acetate (F - J) extracts of Nepeta binaludensis; the results for antibiotics and antifungal as positive controls (K - O)
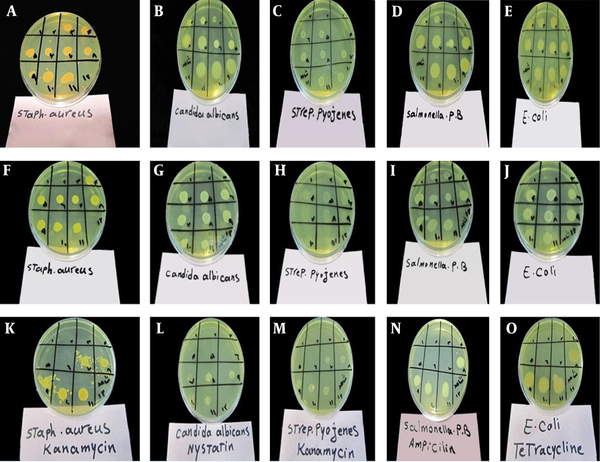
With regards to Table 2, since the initial concentration of ethyl acetate extract (64 mg/mL) was lower than those of the 50% hydro-methanol extract (117 mg/mL) and antibiotics (100 mg/mL), it can be concluded that ethyl acetate extract has significant antibacterial activity against the two Gram-positive bacteria: Staphylococcus aureus and Streptococcus pyogenes and a Gram-negative bacteria Escherichia coli. The effectiveness of 50% hydro-methanol extract on Staphylococcus pyogenes was also considerable. Based on the obtained results, N. binaludensis possesses the compounds with antibacterial properties. These compounds can effectively inhibit the growth of bacteria. Based on previous reports, compounds such as terpenoids and flavonoids have antimicrobial activities (12-14). To determine the nature of the antibacterial effects of the extracts, the MBC/MIC ratio was determined; in all cases, the MBC and MIC values were equal and the ratio was ~1, except for the 50% hydro-methanol extract against Streptococcus pyogenes, which the MBC/MIC ratio was 4. Hence, all the extracts can be considered as bactericidal agents (15).
Crude extracts of plants exhibited in vitro antibacterial, antifungal, and antioxidant activities by many researchers. Nepeta species such as N. cataria L. var. citrodora, N. meyeri Bentham, N. nepetella, N. praetervisa, N. glomerulosa Boiss, N. ciliaris Benth., N. rtanjensis, and N. elliptica Royle ex Benth., showed such properties (16-23). In a study by Kraujalis et al. antioxidant activity of several Nepeta species was evaluated (24). Acetone, methanol, and water were used as solvents for extraction, based on their polarity differences. Results of the studies showed that methanolic extracts have significant antioxidant activities, in comparison with the less polar acetone extracts, and the best results belonged to the extracts of N. cataria. Lee et al. reported a relationship between antioxidant properties of Nepeta species and the presence of phenolic acids, particularly rosmarinic and caffeic acids, in the extract (25). A few reports are also available on biological activities of Nepeta binaludensis. In a study, antimicrobial activity of the essential oil of N. binaludensis was evaluated. Based on the mentioned report, the essential oil showed moderate antimicrobial activity against Staphylococcus aureus, Bacillus cereus, Escherichia coli and Candida albicans. The essential oil had no effects on Pseudomonas aeruginosa (5).
In the other study, Tundis et al. evaluated the antioxidant activity of various extracts of N. binaludensis. They reported that the extracts prepared by polar solvents such as methanol and n-butanol show better results than the other extracts used in the study (26). Tayarani et al. evaluated anti-melanogenic and antioxidant activities of different extracts of N. binaludensis. To assess the inhibitory effects of N. binaludensis on melanogenesis, various assays were carried out. They reported the inhibitory effect of the extracts on melanin synthesis without cytotoxic impacts on B16 melanoma cells (27).
4. Conclusions
The results of the current study can be used as a strategy to develop novel antioxidants and antimicrobial drugs. Nowadays, resistance to antimicrobial agents is increasing, and herbal products are the rich sources for antimicrobial agents. The present study demonstrated that the areal parts of N. binaludensis are good sources of such compounds with antioxidant and antibacterial activities.
Acknowledgements
References
-
1.
Rechinger KH. Nepeta in Lamiaceae, Flora Iranica. Austria: Akademische Druc-u; 1982. p. 108-216.
-
2.
Jamzad Z. Nepeta menthoides Boiss. et Buhse and species allied to it in Iran. Iran J Bot. 1991;5(1):17-27.
-
3.
Nadjafi F, Koocheki A, Honermeier B, Asili J. Autecology, Ethnomedicinal and Phytochemical Studies of Nepeta binaludensis Jamzad a Highly Endangered Medicinal Plant of Iran. J Essential Oil Bearing Plants. 2009;12(1):97-110. https://doi.org/10.1080/0972060x.2009.10643699.
-
4.
Rustaiyan A, Nadji K. Composition of the essential oils of Nepeta ispahanica Boiss. and Nepeta binaludensis Jamzad from Iran. Flavour Fragrance J. 1999;14(1):35-7.
-
5.
Mohammadpour N, Emami SA, Asili J. Identification of Volatile Oil Components ofNepeta binaludensisJamzad by GC-MS and13C-NMR Methods and Evaluation of its Antimicrobial Activity. J Essential Oil Bearing Plants. 2013;16(1):102-7. https://doi.org/10.1080/0972060x.2013.764206.
-
6.
Nadjafi F, Koocheki A, Rezvani Moghaddam P, Honermeier B. First experiments on cultivation of Nepeta binaludensis Jamzad-an example of domestication of a highly endangered medicinal plant of Iran. J Med Spice Plants. 2012;17(2):64-71.
-
7.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticulture. 1965;16(3):144-58.
-
8.
Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Analysis. 2002;10(3).
-
9.
Bondet V, Brand-Williams W, Berset C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH.Free Radical Method. LWT Food Sci Technol. 1997;30(6):609-15. https://doi.org/10.1006/fstl.1997.0240.
-
10.
Harley JP. Laboratory exercises in microbiology. McGraw-Hill Science, Engineering & Mathematics; 2004.
-
11.
Das NP, Pereira TA. Effects of flavonoids on thermal autoxidation of palm oil: Structure-activity relationships. J Am Oil Chemists' Soc. 1990;67(4):255-8. https://doi.org/10.1007/bf02540652.
-
12.
Siddiqui NU, Kapoor A, Srivastava M, Sharma S, Aslam M. Structure - activity relationship between terpenoids and antimicrobial activity. Biosci Biotechnol Res Asia. 2005;3(1).
-
13.
Tereschuk ML, Riera MV, Castro GR, Abdala LR. Antimicrobial activity of flavonoids from leaves of Tagetes minuta. J Ethnopharmacol. 1997;56(3):227-32. https://doi.org/10.1016/s0378-8741(97)00038-x.
-
14.
Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56(1):3-12. https://doi.org/10.1016/s0168-1605(00)00218-x.
-
15.
Levison ME. Pharmacodynamics of antimicrobial drugs. Infect Dis Clin. 2004;18(3):451-65.
-
16.
Modnicki D, Tokar M, Klimek B. Flavonoids and phenolic acids of Nepeta cataria L. var. citriodora (Becker) Balb. (Lamiaceae). Acta Pol Pharm. 2007;64(3):247-52. [PubMed ID: 17695148].
-
17.
Cigremis Y, Ulukanli Z, Ilcim A, Akgoz M. In vitro antioxidant and antimicrobial assays of acetone extracts from Nepeta meyeri Bentham. Eur Rev Med Pharmacol Sci. 2010;14(8):661-8.
-
18.
Seladji M, Bekhechi C, Beddou F, Dib H, Bendimerad N. Antioxidant activity and phytochemical screening of Nepeta nepetella aqueous and methanolic extracts from Algeria. J Appl Pharm Sci. 2014;(4):12-6.
-
19.
Fareed G, Afza N, Mali A, Fareed N, Lateef M, Iqbal L, et al. Phytochemical screening, total phenolic contents and biological evaluation of aerial parts of Nepeta praetervisa. J Chem Soc Pak. 2013;35:1364-8.
-
20.
Nezhadali A, Masrornia M, Bari H, Akbarpour M, Joharchi MR, Nakhaei-Moghadam M. Essential Oil Composition and Antibacterial Activity ofNepeta glomerulosaBoiss from Iran. J Essential Oil Bearing Plants. 2011;14(2):241-4. https://doi.org/10.1080/0972060x.2011.10643927.
-
21.
Gautam SS, Navneet Kumar S, Prabhat M. Screening of antibacterial activity of nepeta ciliaris benth against respiratory tract pathogens. Kathmandu Univers J Sci Eng Technol. 2012;8:100-3.
-
22.
Grbic-Ljaljevic M, Stupar M, Vukojevic J, Sokovic M, Misic D, Grubisic D, et al. Antifungal activity of Nepeta rtanjensis essential oil. J Serbian Chem Soc. 2008;73(10):961-5. https://doi.org/10.2298/jsc0810961g.
-
23.
Kumar V, Mathela CS, Tewari G, Singh D. Antifungal activity of Nepeta elliptica Royle ex Benth. oil and its major constituent (7R)-trans,trans-nepetalactone: A comparative study. Industr Crops Prod. 2014;55:70-4. https://doi.org/10.1016/j.indcrop.2014.02.003.
-
24.
Kraujalis P, Venskutonis PR, Ragazinskiene O. Antioxidant activities and phenolic composition of extracts from Nepeta plant species. FOODBAL; 2011.
-
25.
Lee SY, Lee CY, Eom SH, Kim YK, Park NI, Park SU. Rosmarinic acid production from transformed root cultures of Nepeta cataria L. Sci Res Essays. 2010;5(10):1122-6.
-
26.
Tundis R, Nadjafi F, Menichini F. Angiotensin-converting enzyme inhibitory activity and antioxidant properties of Nepeta crassifolia Boiss & Buhse and Nepeta binaludensis Jamzad. Phytother Res. 2013;27(4):572-80. [PubMed ID: 22693035]. https://doi.org/10.1002/ptr.4757.
-
27.
Tayarani-Najaran Z, Akaberi M, Vatani M, Emami SA. Evaluation of antioxidant and anti-melanogenic activities of different extracts from aerial parts of Nepeta binaludensis Jamzad in murine melanoma B16F10 cells. Iran J Basic Med Sci. 2016;19(6):662-9. [PubMed ID: 27482348]. [PubMed Central ID: PMC4951606].
