Abstract
Background:
Side effects of chemical preservatives and drug resistance have raised interests in the use of natural preservatives derived from plants.Objectives:
The aim of this study was to examine possible antibacterial effects of Mentha spicata L., Cuminum cyminum L. and Mentha longifolia L. essential oils (EOs) individually and in combination with sodium benzoate against Escherichia coli O157:H7 and Listeria monocytogenes.Methods:
In this experimental study, the EOs were obtained and analyzed by gas chromatography mass spectrometry. Disc diffusion and broth microdilution methods were used for in vitro antibacterial screening in triplicate. Data analysis was performed by the SPSS software using ANOVA and independent sample t-test.Results:
Statistical analysis showed a significant difference between different antibacterial effects of EOs individually and in combination with sodium benzoate. Considering the individual effects of these factors, the antibacterial effect of sodium benzoate and Cuminum cyminum essential oil were the highest against E. coli O157:H7 and L. monocytogenes, respectively. These results are relatively consistent with the disc diffusion test. The antibacterial effects of sodium benzoate in combination with EOs showed significant differences in comparison to sodium benzoate effect individually in most situations (P < 0.05).Conclusions:
The results indicated that these EOs have a good antibacterial activity and combined with sodium benzoate could reduce the use of sodium benzoate as a chemical preservative in food, cosmetic, and drug products, which can decrease the possible side effects of it.Keywords
Antibacterial Essential Oil Sodium Benzoate Escherichia coli O157:H7 Listeria monocytogenes
1. Background
Most of the consumers concern that the side effects of chemical and synthetic preservatives and calls for a replacement with their natural counterparts are increasing. The resistance of pathogens to antimicrobial compounds has lethal effects as the development of drug resistance outpaces the development of new drugs (1). Recently, there has been much interest in novel biological active and anti-microbial active extracts of natural products, particularly those found in medicinal plants (2). Essential oils (EOs) are volatile aromatic components that are obtained from different parts of plants (3). Many researchers have reported the antimicrobial, antifungal, and antioxidant properties of EOs (1, 2, 4). The action mechanism of the EOs relates to their chemical components that have the ability to easily diffuse across the cell membrane to induce biological reactions. Also, the hydrophobicity of the EOs is the main properties responsible for the distribution of bacterial structures, increasing their permeability. Therefore, EOs are valuable natural products that can be used as raw materials in many fields, including perfumes, cosmetics, aromatherapy, phytotherapy, spices, and nutrition (5). Mentha spicata (Lamiaceae family) grows all over the world and this plant is widely employed as a flavoring agent in several foods, also cosmetic, confectionary, and pharmaceutical industries (6). The wild mint (Mentha longifolia L. family Lamiaceae) is an herb with a wide range of pharmacological properties such as antimicrobial properties, and effects on gastrointestinal and nervous system (7). Cuminum cyminum (cumin or Jeera) is another medicinal herb belonging to the Apiaceae family, which is commonly used for flavoring foods and medical preparation. It has excellent antioxidant, antibacterial, and antifungal properties (8-10). Sodium benzoate is commonly used as a preservative in liquid pharmaceutical preparations and food industry as antimicrobial agents (11). The majority of preservatives used today are artificial rather than natural. Several of them are toxic and several others have potentially life-threatening side effects (12). Benzoates have been suspected to cause allergies, asthma, and skin rashes (13). Owing to the harmful effects of artificial preservatives, the use of natural preservatives has been recommended for better therapeutic efficacy and safety. Natural substances such as EOs can serve as beneficial alternatives (12). L. monocytogenes is one of the most food-borne pathogens and one of the major causes of infections in developing countries that has been found in different environments (14). E. coli O157:H7 has emerged in recent years as an important pathogen that can cause diarrhea or hemorrhagic colitis in humans. Owing to the morbidities and mortalities, this pathogen is now considered a major public health problem in the world (15). Essential oils have already proven to exert strong synergistic effects when used in combination with artificial and less effective preservatives (16).
2. Objectives
Therefore the objective of this study was to evaluate the effects of the EOs individually and in combination with sodium benzoate against E. coli O157:H7 and L. monocytogenes.
3. Methods
3.1. Preparation of Essential Oils and Microbial Strains
The EOs were obtained from Barij Esans Company (Kashan, Iran). Lyophilized bacterial strains of E. coli O157:H7 ATCC 43895 and L. monocytogenes ATCC 19113 were purchased from Pasteur Institute (Tehran, Iran). The lyophilized culture was grown in a tube containing brain and heart infusion (BHI) broth (Merck, Germany) twice and incubated at 37°C for 24 hours.
3.2. Chemical Analysis of Essential Oils
The EOs were analyzed by gas chromatography (GC) (Agilent 7890S, UK) and also analyzed by gas chromatography-mass spectrometry (GC-MS) (5975C, UK) equipped with an HP-5MS capillary column (30 m × 0.25 mm ID × 0.25 µm film thickness). The helium was the carrier gas with 1 mL/min split ratio. The column temperature was at first 40°C, and then gradually increased to 300°C at a 5°C/min rate and held for 1 min. The MS was scanned in the electron impact way using 70e-V ionization energy. The detection of the major compounds of EOs was based on the comparison between their retention indices, standard mass spectral fragmentation pattern, and the National Institute of Standards and Technology. Then the percentage of them was calculated from GC peak areas (17).
3.3. Disc Diffusion Assay
The antibacterial activity of EOs and sodium benzoate was investigated by agar disc diffusion assay, according to the guideline of the Clinical and Laboratory Standards Institute (CLSI) (18). Sterile blank discs (Padtan Teb, Iran) 6 mm in diameter were impregnated with 30 µL of essential oil and sodium benzoate (equivalent to minimum inhibitory concentration) and aseptically placed on the inoculated Mueller-Hinton agar (MHA, Quelab, Canada) medium with the bacteria, which their turbidity were equal to 0.5 McFarland. The solvent of EOs (dimethyl sulfoxide (DMSO)) only was used as negative control. Erythromycin (15 µg/disc), streptomycin (10 µg), gentamicin (10 µg), and ciprofloxacin (5 µg) (Padtan Teb, Iran) were used as the positive control. The inoculated plates were incubated overnight at 37°C for 24 hours. The antibacterial effect was evaluated by measuring the inhibition zone against the organisms (mm). Each experiment was performed in triplicate.
3.4. Micro-Well Dilution Assay
For the preparation microbial inoculants of bacteria (5 × 106 CFU/mL), one isolated colony of each bacterium were inoculated into a tube containing 5 mL of Mueller-Hinton Broth (MHB, Quelab, Canada) medium and incubated at 37°C for 20 hours. The second transfer of 0.1 mL of cultures in 5 mL of MHB was grown at 37°C for 20 hours. After twice incubation, the number of bacterial colonies was enumerated by serial dilution and plating on MHA medium in triplicate. The broth microdilution assay was used to examine the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) in sterile 96-well microplates in triplicate (19, 20). First, 10% (v/v) dimethyl sulfoxide (DMSO) (Samchun, South Korea) as an emulsifier of the EOs was added to Mueller-Hinton Broth medium. Different concentrations of the EOs (0.01% to 4.4%) were set up in MHB medium, containing DMSO. in brief, 160 µL of MHB containing DMSO, 20 µL of different concentrations of the EOs or sodium benzoate (0.01% to 4.4%) and 20 µL of inoculum diluted in MHB with 5 × 106 CFU/mL of the bacteria were added to each well to achieve a concentration of 105 CFU/well. The last well containing 180 µL of MHB containing DMSO and 20 µL of bacterial inoculum without essential oil or sodium benzoate was designed as the positive control sample. For negative control, un-inoculated MHB containing DMSO was used. The contents of each well were mixed on a shaker rotator at 300 rpm for 20 seconds and incubated at 37°C for 24 hours. The plates were placed in a wet container to ensure that the bacteria did not get dehydrated during incubation. To determine combination effects of sodium benzoate with EOs, the procedure presented above was used, but 140 µL of MHB containing DMSO, 20 µL of different concentrations of sodium benzoate (0.01% to 4.4%) with 20 µL of equivalent of ½ MIC of the EOs for each bacterium and 20 µL of the bacterial inoculum were added to each well. The concentration of the first well without turbidity was reported as MIC. The MIC was defined as the lowest concentration of the essential oil or sodium benzoate that prevented the growth of the inoculated bacteria. To determine MBC, the contents of the non-growth wells were cultured on MHA plates and incubated at 37°C for 24 hours. The MBC was considered the lowest concentration in which no bacterial growth was detectable on the plates.
3.5. Statistical Analysis
All data were declared as means ± standard deviations (SD) of triplicate measurements. Statistical analysis of data was performed using analysis of variance (ANOVA) by SPSS 16 software and mean comparison was done by Duncan test. Differences were considered significant when P ≤ 0.05. Also, Independent Sample t-test was used to compare the results of E. coli O157:H7and L. monocytogenes to each other.
4. Results
4.1. Chemical Composition of Essential Oils
The oils were liquid and their odors were acceptable. Results of GC-MS analyses (Table 1) showed that the chemical compositions of them were totally different. The main components were Cumin aldehyde (28.24% of the total essential oil), carvone (58.89%) and pulegone (66.95%) in C. cyminum, M. spicata and M. longifolia essential oils, respectively. These results were reported also by some researches (21-24). It can be observed that monoterpenes (74.17%) are majority compound in C. cyminum. Also, oxygenated monoterpenes were found as the majority compound in M. spicata (63.06%) and M. longifolia (89.47%) EOs.
Chemical Composition of Cuminum cyminum (A), Mentha longifolia (B), and Mentha spicata (C) Essential Oils
| Component | Composition, % | |||
|---|---|---|---|---|
| KIa | A | B | C | |
| Sabinene | 975 | 0.38 | 0.35 | |
| β-Pinene | 979 | 9.14 | 0.7 | 0.73 |
| β-Myrcene | 991 | 0.51 | 0.25 | 0.47 |
| α-Terpinene | 1017 | 0.17 | ||
| o-Cymene | 1025 | 13.78 | ||
| Limonene | 1029 | 1.07 | 27.34 | |
| β-phellandrene | 1030 | 0.6 | ||
| 1, 8-Cineole | 1031 | 0.04 | 4.06 | 0.13 |
| Trans-Para-2, 8 Menthadien | 1138 | 0.07 | ||
| γ-Terpinene | 1060 | 21.39 | 0.04 | 0.04 |
| 3, 8-Menthadien | 1073 | 0.42 | ||
| α-Terpinolene | 1089 | 0.07 | 0.02 | |
| Menthone | 1163 | 2.71 | ||
| Menthofuran | 1164 | 10.89 | ||
| Borneol | 1169 | 0.69 | ||
| Cis-isopulegon | 1172 | 1.30 | ||
| Menthol | 1172 | 0.06 | ||
| 4-Terpineole | 1177 | 0.1 | 0.11 | 0.1 |
| Dihydrocarvone | 1193 | 1.07 | ||
| Myrtenol | 1196 | 0.04 | ||
| Trans-Pulegone | 1215 | 0.19 | ||
| Cic-Pulegone | 1229 | 0.2 | ||
| Pulegone | 1237 | 66.95 | 2.81 | |
| Cumin aldehyde | 1242 | 28.24 | ||
| Carvone | 1243 | 0.63 | 58.89 | |
| Cic-Piperitenone oxide | 1254 | 0.54 | ||
| Bornyl acetate | 1289 | 0.06 | ||
| Carvacrol | 1299 | 0.17 | ||
| α-Cubebene | 1351 | 0.02 | ||
| Piperitenone oxide | 1369 | 1.2 | ||
| Trans-β-Damascenone | 1385 | 0.02 | ||
| Cis-Jasmone | 1393 | 0.05 | ||
| Trans-Caryophyllene | 1419 | 0.2 | 0.84 | 1.85 |
| Trans-α-Bergamotene | 1435 | 0.06 | ||
| α-Humulene | 1455 | 0.3 | ||
| Trans-β-Farnesene | 1457 | 0.27 | ||
| β-Acoradiene | 1471 | 1.68 | ||
| Curcumene | 1481 | 0.04 | ||
| Germacrene-D | 1485 | 0.3 | 0.11 | |
| Caryophyllene-oxide | 1583 | 0.12 | 0.15 | |
| Bicyclogermacrene | 1500 | 0.17 | ||
| Carotol | 1595 | 0.43 | ||
| λ-Amorphene | 1512 | 0.01 | ||
| Monoterpenes | 74.17 | 2.97 | 29.02 | |
| Oxygenated monoterpenes | 0.08 | 89.47 | 63.06 | |
| Sesquiterpenes | 2.27 | 1.31 | 2.41 | |
| Oxygenated sesquiterpenes | 0.58 | |||
4.2. Antibacterial Activity
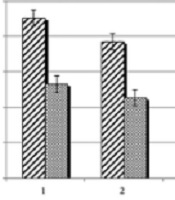
The results of the antibacterial activity of the EOs and sodium benzoate on E. coli O157:H7and L. monocytogenes by the disc diffusion method are shown based on the diameter of the inhibition zone in Table 2. The results of broth microdilution method are presented in Tables 3 and 4, and Figure 1 shows means and standard deviations of MIC and MBC of the EOs individually and in combination with sodium benzoate, respectively. Statistical analysis using analysis of variance showed a significant difference between different antibacterial effects of EOs individually and in combination with sodium benzoate on tested bacteria. Considering the individual effects, the antibacterial effect of sodium benzoate against E. coli O157:H7 was the highest and then Mentha longifolia, Cuminum cyminum, and Mentha spicata essential oils indicated antibacterial activity, respectively. These results are relatively consistent with the disc diffusion test. However, the growth inhibitory zone of sodium benzoate was lower that may be because sodium benzoate placed on the disc was obtained from MIC dilution, while the oils were placed directly. Also, the combined effect of sodium benzoate with Mentha longifolia essential oil was the strongest against E. coli. The effects of sodium benzoate combined with Mentha longifolia and Mentha spicata essential oils on E. coli showed significant differences compared with sodium benzoate effect individually (P <0.05). About L. monocytogenes, given the individual effects of affected factors, Cuminum cyminum essential oil was the highest and then Mentha spicata, Mentha longifolia, and sodium benzoate indicated antibacterial activity, respectively. These results are relatively consistent with the disc diffusion test. Moreover, the combined effect of sodium benzoate with Cuminum cyminum essential oil was strong against L. monocytogenes. The effects of sodium benzoate in combination with EOs on L. monocytogenes showed significant differences compared with sodium benzoate effect individually (P < 0.05). The comparison of the results of two bacteria using independent sample t-test showed effects of Mentha spicata, Cuminum cyminum essential oils individually and sodium benzoate in combination with Cuminum cyminum on L. monocytogenes is significantly higher than E. coli (P < 0.05). On the other hand, effects of sodium benzoate alone and in combination with Mentha longifolia and Mentha spicata essential oils on E. coli is significantly higher than L. monocytogenes. However, the effect of Mentha longifolia essential oil on studied bacteria was not significant (P > 0.05). Based on the results of MBC (Tables 3, 4), these factors in the studied concentrations had not bactericidal effect against L. monocytogenes, especially individually. Nevertheless, sodium benzoate in combination with oils showed a synergistic inhibitory effect. The results showed the medicinal plant EOs could prevent the growth of the bacteria. However, to obtain more potent synergistic effects, a combination of sodium benzoate with EOs and other natural antimicrobials is suggested.
Antibacterial Effect of the Essential Oils, Sodium Benzoate, and Standard Antibiotic Discs by Agar Disc Diffusion Assay
| Inhibition Zone in Diameter, mm | Cuminum cyminum | Mentha longifolia | Mentha spicata | Sodium Benzoate (MIC) | Erythromycin, 15 µg | Streptomycin, 10 µg | Ciprofloxacin, 5 µg | Gentamicin, 10 µg | DMSO |
|---|---|---|---|---|---|---|---|---|---|
| E. coli O157H7 | 11 | 14 | 10 | 13 | 12 | 12 | 31 | 15 | R |
| L. monocytogenes | 14 | 9 | 11 | 12 | 28 | 18 | 30 | 31 | R |
Mean and Standard Deviation of MIC and MBC of Sodium Benzoate Individually and in Combination with Essential Oils Against E. coli O157:H7 and L. monocytogenesa
| Bacteria | Sodium Benzoate + ½ MIC of Essential Oils | |||
|---|---|---|---|---|
| Sodium Benzoate | Sodium Benzoate + ½ MIC of Cuminum cyminum | Sodium Benzoate + ½ MIC of Mentha longifolia | Sodium Benzoate + ½ MIC of Mentha spicata | |
| E. coli O157H7 | ||||
| MIC, % | 1.06 ± 0.11 | 0.93 ± 0.11 | 0.46 ± 0.11 | 0.6 ± 0.0 |
| MBC, % | 4.26 ± 0.11 | 4.2 ± 0.0 | 4.06 ± 0.11 | 3.26 ± 0.11 |
| L. monocytogenes | ||||
| MIC, % | 1.8 ± 0.0 | 0.36 ± 0.05 | 0.93 ± 0.11 | 1.13 ± 0.11 |
| MBC, % | Nb | 3.26 ± 0.11 | Nb | 3.26 ± 0.11 |
Mean and standard deviation of MIC of essential oils and sodium benzoate individually and combined against E. coli O157:H7 and L. monocytogenes. 1, Mentha spicata; 2, Cuminum cyminum; 3, Mentha longifolia; 4, sodium benzoate; 5, sodium benzoate + ½ MIC of Mentha spicata; 6, sodium benzoate + ½ MIC of Cuminum cyminum; 7, sodium benzoate + ½ MIC of Mentha longifolia.

5. Discussion
In the recent years, owing to the high interest and demand of consumers to use natural sources from the industries of pharmaceutical and cosmetics, fragrance, and food flavoring manufacturers, the use of medicinal plants, especially herbal EOs has gained huge attraction (25). The indiscriminate use of chemical antimicrobial drugs for microbial infections has developed multiple resistances. Therefore, nowadays natural products are used in medicine and food industry as antimicrobial activities (26). Plant EOs are potential sources of new antimicrobial compounds (27). The composition of herbal EOs can be variable depending on several factors such as geographic region, harvest time, variety, age of the plant, extraction method, and the type of culture (28). Variation in the chemical compound of EOs could affect their biological activities. Therefore, despite the various studies on the chemical composition and antimicrobial effect of the EOs, it was important to determine the chemical compounds of EOs to correlate with their antimicrobial effects (29). The profiles of the studied EOs about most abundant chemical compounds were in agreement with previous studies (21-24). Monoterpenes and oxygenated monoterpenes were the main compounds of the studied EOs. The results of previous researches have shown that these compounds have a degree of antibacterial activities (30, 31). In spite of the strong inhibitory effects of EOs against bacterial pathogens, the antimicrobial effects of EOs in food and oral drugs can change the taste of them. Thus its application as a preservative is limited (32). Leistner and Goris recommended that food and drug preservation by several preservatives in low amount is better compared to preservation by a high amount of a single preservative (33). Therefore, to establish the efficiency of natural antimicrobials, they must be evaluated alone and in combination with other antimicrobial agents to detect whether there are synergistic effects (34). Nowadays, extensive efforts have been taken to use the lowest concentrations of EOs with other antimicrobial agents to receive more strong synergistic effects (16, 17, 35). For example, the antibacterial effect of M. spicata essential oil and nisin has previously been reported against L. monocytogenes (17). In a study, monolaurin spearmint oil combination has been indicated synergistic inhibitory effect on E. coli O157:H7 (35). In this study, we found a significant (P < 0.05) synergistic inhibitory effect of the EOs and sodium benzoate against E. coli O157:H7 and L. monocytogenes. There is evidence that Gram-positive bacteria are more sensitive to plant oils than Gram-negative bacteria. Results obtained in this study showed that the EOs do not have selective antimicrobial activity on the basis of the cell-wall differences of the bacteria as reported previously (36). This variation in sensitivity between the two bacteria may be a result of differences in the cell membranes or metabolism system (16). This result is supported by findings of Nikšić et al. that proved the strongest antibacterial activity effect of M. longifolia oil was against Gram-negative strains, including E. coli, P. aeruginosa, and S. enterica (37). Moreover, Gulluce et al. indicated the essential oil of M. longifolia collected from Turkey had strong antibacterial activity against numerous bacteria such as L. monocytogenes, S. typhimurium, and E. coli O157:H7 (38). According to Steffanini et al. essential oil of C. cyminum was active against different Gram-negative bacteria, including E. coli, P. aeruginosa and Salmonella sp. (39). Furthermore, previous antibacterial studies showed that M. spicata EO had an antibacterial effect on the growth of Gram-negative and Gram-positive bacteria (40). The antibacterial activity could be influenced by terpene hydrocarbons, but other components can contribute to this activity (24). The antimicrobial activity of the M. spicata essential oil could be associated to carvone and limonene. The mechanism of carvone action is related to the destabilization of the phospholipid bilayer structure, interaction with enzymes and proteins of the membrane, and acts as a proton exchanger to reduce the pH among the membrane (17). Also, pulegone and cumin aldehyde, the main compounds of the M. longifolia and C. cyminum, respectively are chiefly responsible for most of their antibacterial activity and pharmacological effects (21-24). Nonetheless, other important compounds may be found in EOs that are not abundant (24). The results of this study demonstrate more efficient antimicrobial action of preservatives when used in combination with other preservatives than when used individually, which indicates the possibility of avoiding the use of higher concentrations of sodium benzoate that could lead to accumulation of toxic products. Therefore, to observe a significant effect on E. coli O157:H7 and L. monocytogenes, a combination of EOs with other antimicrobials such as sodium benzoate is suggested. However, further in vivo studies are necessary for pharmaceuticals and natural therapies of infectious diseases in the human.
Acknowledgements
References
-
1.
Naveed R, Hussain I, Tawab A, Tariq M, Rahman M, Hameed S, et al. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Complement Altern Med. 2013;13:265. [PubMed ID: 24119438]. [PubMed Central ID: PMC3853939]. https://doi.org/10.1186/1472-6882-13-265.
-
2.
Gavanji S, Mohammadi E, Larki B, Bakhtari A. Antimicrobial and cytotoxic evaluation of some herbal essential oils in comparison with common antibiotics in bioassay condition. Integr Med Res. 2014;3(3):142-52. [PubMed ID: 28664090]. [PubMed Central ID: PMC5481736]. https://doi.org/10.1016/j.imr.2014.07.001.
-
3.
Burt S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94(3):223-53. [PubMed ID: 15246235]. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022.
-
4.
Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, et al. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91(4):621-32. https://doi.org/10.1016/j.foodchem.2004.06.031.
-
5.
Lahlou M. Essential oils and fragrance compounds: Bioactivity and mechanisms of action. Flavour Frag J. 2004;19(2):159-65. https://doi.org/10.1002/ffj.1288.
-
6.
Kumar P, Mishra S, Malik A, Satya S. Insecticidal properties of Mentha species: A review. Ind Crops Prod. 2011;34(1):802-17. https://doi.org/10.1016/j.indcrop.2011.02.019.
-
7.
Mikaili P, Mojaverrostami S, Moloudizargari M, Aghajanshakeri S. Pharmacological and therapeutic effects of Mentha Longifolia L. and its main constituent, menthol. Anc Sci Life. 2013;33(2):131-8. [PubMed ID: 25284948]. [PubMed Central ID: PMC4171855]. https://doi.org/10.4103/0257-7941.139059.
-
8.
Skrovankova S, Misurcova L, Machu L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012;67:75-139. [PubMed ID: 23034115]. https://doi.org/10.1016/B978-0-12-394598-3.00003-4.
-
9.
Wanner J, Bail S, Jirovetz L, Buchbauer G, Schmidt E, Gochev V, et al. Chemical composition and antimicrobial activity of cumin oil (Cuminum cyminum, Apiaceae). Nat Prod Commun. 2010;5(9):1355-8. [PubMed ID: 20922990].
-
10.
Pai MB, Prashant GM, Murlikrishna KS, Shivakumar KM, Chandu GN. Antifungal efficacy of Punica granatum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: An in vitro study. Indian J Dent Res. 2010;21(3):334-6. [PubMed ID: 20930339]. https://doi.org/10.4103/0970-9290.70792.
-
11.
Boukarim C, Abou Jaoude S, Bahnam R, Barada R, Kyriacos S. Preservatives in liquid pharmaceutical preparations. Drug Test Anal. 2009;1(3):146-8. [PubMed ID: 20355189]. https://doi.org/10.1002/dta.28.
-
12.
Anand SP, Sati N. Artificial preservatives and their harmful effects: looking toward nature for safer alternatives. Int J Pharm Sci Res. 2013;4(7):2496.
-
13.
Sharma S. Food preservatives and their harmful effects. Int J Sci Res Public. 2015;5(4):1-2.
-
14.
Miettinen H, Wirtanen G. Prevalence and location of Listeria monocytogenes in farmed rainbow trout. Int J Food Microbiol. 2005;104(2):135-43. [PubMed ID: 15993967]. https://doi.org/10.1016/j.ijfoodmicro.2005.01.013.
-
15.
Buchanan A. Foodborne disease significance of Escherichia coli O157: H7 and other enterohemorrhagic E. coli. Food Technol. 1997.
-
16.
Pajohi MR, Tajik H, Farshid AA, Hadian M. Synergistic antibacterial activity of the essential oil of Cuminum cyminum L. seed and nisin in a food model. J Appl Microbiol. 2011;110(4):943-51. [PubMed ID: 21226797]. https://doi.org/10.1111/j.1365-2672.2011.04946.x.
-
17.
Moosavy MH, Shahbazi Y, Shavisi N. The combined effect of mentha spicata essential oil and nisin against Listeria monocytogenes. Pharm Sci. 2015;21(4):178-83. https://doi.org/10.15171/ps.2015.34.
-
18.
Wayne P. Clinical and Laboratory Standards Institute (CLSI) performance standards for antimicrobial disk diffusion susceptibility tests. 19th ed. CLSI document M100-S19; 2009.
-
19.
Wayne PA. Clinical and Laboratory Standards Institute (CLSI) method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 8th ed. USA: CLSI document M07-A8; 2009.
-
20.
Razavi Rohani SM, Moradi M, Mehdizadeh T, Saei-Dehkordi SS, Griffiths MW. The effect of nisin and garlic (Allium sativum L.) essential oil separately and in combination on the growth of Listeria monocytogenes. LWT Food Sci Technol. 2011;44(10):2260-5. https://doi.org/10.1016/j.lwt.2011.07.020.
-
21.
Gachkar L, Yadegari D, Rezaei M, Taghizadeh M, Astaneh S, Rasooli I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007;102(3):898-904. https://doi.org/10.1016/j.foodchem.2006.06.035.
-
22.
Dussault D, Vu KD, Lacroix M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014;96(1):514-20. [PubMed ID: 24012976]. https://doi.org/10.1016/j.meatsci.2013.08.015.
-
23.
Govindarajan M, Sivakumar R, Rajeswari M, Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitol Res. 2012;110(5):2023-32. [PubMed ID: 22139403]. https://doi.org/10.1007/s00436-011-2731-7.
-
24.
Mkaddem M, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Romdhane M. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J Food Sci. 2009;74(7):M358-63. [PubMed ID: 19895481]. https://doi.org/10.1111/j.1750-3841.2009.01272.x.
-
25.
Sağdıç O, Kuşçu A, Özcan M, Özçelik S. Effects of Turkish spice extracts at various concentrations on the growth of Escherichia coli O157:H7. Food Microbiol. 2002;19(5):473-80. https://doi.org/10.1006/fmic.2002.0494.
-
26.
Martins MR, Tinoco MT, Almeida AS, Cruz-Morais. J. Chemical composition, antioxidant and antimicrobial properties of three essential oils from Portuguese flora. J Pharmacogn. 2012;3(1):39-44.
-
27.
Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-82. [PubMed ID: 10515903]. [PubMed Central ID: PMC88925]. https://doi.org/10.1128/CMR.12.4.564.
-
28.
Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21(1):33-42. https://doi.org/10.1016/s0740-0020(03)00046-7.
-
29.
Lin K, Yeh S, Lin M, Shih M, Yang K, Hwang S. Major chemotypes and antioxidative activity of the leaf essential oils of Cinnamomum osmophloeum Kaneh. From a clonal orchard. Food Chem. 2007;105(1):133-9. https://doi.org/10.1016/j.foodchem.2007.03.051.
-
30.
Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C. 2007;62(7-8):507-13. [PubMed ID: 17913064]. https://doi.org/10.1515/znc-2007-7-808.
-
31.
Zengin H, Baysal AH. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19(11):17773-98. [PubMed ID: 25372394]. [PubMed Central ID: PMC6272013]. https://doi.org/10.3390/molecules191117773.
-
32.
Valero M, Giner MJ. Effects of antimicrobial components of essential oils on growth of Bacillus cereus INRA L2104 in and the sensory qualities of carrot broth. Int J Food Microbiol. 2006;106(1):90-4. [PubMed ID: 16213622]. https://doi.org/10.1016/j.ijfoodmicro.2005.06.011.
-
33.
Leistner L, Gorris LGM. Food preservation by hurdle technology. Trends Food Sci Technol. 1995;6(2):41-6. https://doi.org/10.1016/s0924-2244(00)88941-4.
-
34.
Pol IE, Krommer J, Smid EJ. Bioenergetic consequences of nisin combined with carvacrol towards Bacillus cereus. Inn Food Sci Emerg Technol. 2002;3(1):55-61. https://doi.org/10.1016/s1466-8564(01)00055-8.
-
35.
Neyriz Nagadehi M, Razavi Rohani SM, Karim G, Razavilar V, Zeynali A, Delshad R. The effect of monolaurin in combination with mentha pulegium L. And mentha spicata L. Essential oils on bacillus cereus and e. Coli o157:H7: In vitro study. Vet Clin Pathol (Vet J Tabriz). 2010;3(4 (12)):657-66.
-
36.
Sahin F, Karaman I, Gulluce M, Ogutcu H, Sengul M, Adiguzel A, et al. Evaluation of antimicrobial activities of Satureja hortensis L. J Ethnopharmacol. 2003;87(1):61-5. [PubMed ID: 12787955]. https://doi.org/10.1016/S0378-8741(03)00110-7.
-
37.
Nikšić H, Kovač Bešović E, Makarević E, Durić K. Chemical composition, antimicrobial and antioxidant properties of Mentha longifolia (L.) Huds. essential oil. J Health Sci. 2012;2(3):192-200. https://doi.org/10.17532/jhsci.2012.38.
-
38.
Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, et al. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103(4):1449-56. https://doi.org/10.1016/j.foodchem.2006.10.061.
-
39.
Stefanini MB, Figueiredo RO, Ming LC, Júnior AF. Antimicrobial activity of the essential oils of some spice herbs. InInternational Conference on Medicinal and Aromatic Plants (Part II). 2001. p. 215-6.
-
40.
Shahbazi Y. Chemical composition and in vitro antibacterial activity of Mentha spicata essential oil against common food-borne pathogenic bacteria. J Pathog. 2015;2015:916305. [PubMed ID: 26351584]. [PubMed Central ID: PMC4553199]. https://doi.org/10.1155/2015/916305.