Abstract
Background:
Arsenic is a potent environmental toxic agent and leads to the development of various hazardous effects on human health. All human populations are exposed to arsenic and its compounds through occupational or environmental processes. Epidemiological studies also suggested that lentils through a numerous biological activities including antioxidant, anticancer, angiotensin-converting enzyme inhibition, as well as reducing blood lipid and the risk of cardiovascular diseases admit protection against chronic diseases.Objectives:
The current study aimed at assessing the protective effect of the red lentil extract (RLE) on sodium arsenite (SA) induced oxidative stress in animals.Methods:
Twenty-four Wistar male rats were divided into 4 groups. The first group (control group) received normal saline (2 mL/kg) per os for 25 days. Groups 2 - 4 were treated with SA (10 mg/kg) per os for 10 days; and the groups 3 and 4 received 100 and 200 mg/kg of RLE per os for 15 days, respectively, after treatment with SA.Results:
Results showed that total phenolic content in crude RLE was 50.6 mg catechin equivalent/g of dry weight. In SA treated groups, serum activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lipid peroxidation (MDA) in the liver increased. Whereas, the activity of superoxide dismutase (SOD) and catalase and the content of sulfhydryl groups (SH-groups) significantly decreased in liver in the intervention group, compared with those of the control group (P < 0.05). Treatment with RLE reduced the serum level of AST, ALT, and ALP and the level of MDA in the liver, while the activity of the antioxidant enzymes and the level of SH-groups increased.Conclusions:
It can be concluded that RLE restored the activity of antioxidant enzymes and serum markers in SA-treated rats, probably by scavenging free radicals and reducing oxidative stress as an antioxidant.Keywords
1. Background
Widespread contamination by heavy metals and metalloids including arsenic, mercury, cadmium and lead (toxic, non-essential elements) can affect the events in the world (1). Among the heavy metals, due to having potential carcinogenicity in human and its complex metabolism, arsenic is a special pollutant class that can increase hazard to health (2). Also, cellular damage of arsenic are initiated through the generation of free radicals, which cause lipid, protein, and nucleic acid oxidation, and moreover, induce biological complications including carcinogenesis, mutagenesis, aging, and atherosclerosis (3, 4). Besides instability in the structure and function of proteins, covalent interactions occur with proteins on the thiol groups in exposure to arsenic. Due to releasing arsenic compounds via industrial, agricultural, and medical processes into the environment, exposure to them is probable everywhere (5). On the other hand, in many areas of the world consumption of contaminated water with arsenic compounds is prevalent (6). However, it is a major risk factor in individuals exposed to insecticides, acaroids, and soaps in which arsenic compounds are used (7). In particular, sodium arsenite (SA) causes chromosomal breakage and its effects with other substances such as metals are potentiated (7, 8). It is reported that the integrity of the liver of mouse and rat in the administration of SA is interrupted (9). Evidence showed that applying nutritional antioxidants, especially plant isolated compounds, can treat the diseases that are related to oxidative stress by scavenging free radicals and modulating antioxidant defense system (10, 11).
Studies showed that extracted phenolic compounds from edible and non-edible parts of plants have antioxidant activity. By involving the initiation or propagation of oxidizing chain reactions, they display their potentiality to inhibit or delay the oxidation of lipids, proteins, and DNA. Obviously, natural phenolic antioxidants can prevent the attack of oxidative diseases in the body by scavenging reactive oxygen and nitrogen species (12, 13). Epidemiological studies showed a relationship between the intake of foods rich in phenolic compounds and decrease in the prevalence of several oxidative diseases (14, 15). In this regard, a number of researchers confirmed the high antioxidant potential constituents present in plant extracts derived from tannin (16, 17).
The lentil plant, Lens culinaris L., is a member of Leguminoceae family. Legumes seeds are herbal foodstuff potentially rich in phenolic compounds, including condensed tannins (18, 19). The antioxidant activity of phenolic compounds extracted from leguminous seeds is investigated by using several in vitro chemical assays (20-22). Epidemiological studies also suggested the protective activity of lentils against chronic diseases through a number of biological activities including antioxidant, anticancer, angiotensin-converting enzyme inhibition, and reducing blood lipid levels as well as the risk of cardiovascular diseases (23-25). Therefore, the current study aimed at evaluating the antioxidant and antiradical activity of the red lentil extract (RLE) on SA induced oxidative stress in the liver tissue.
2. Methods
2.1. Chemicals
SA (NaAsO2), 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), reduced glutathione (GSH), trichloroacetic acid (TCA), thiobarbituric acid (TBA), bovine serum albumin (BSA), and Bradford reagent were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Assay kits for serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphates (ALP) were obtained from Randox Laboratories Ltd., United Kingdom. All chemicals and the reagents used were analytical grade.
2.2. Plant Material
Red lentil seeds (Lens culinaris L.) were collected from Khuzestan provincial local market, Iran, in April 2015. The seeds were identified at the herbarium of the department of pharmacognosy, school of pharmacy, Ahvaz, Iran.
2.3. Extract Preparation
A 500-g of red lentil seeds was crushed into small pieces and soaked in a 70% aqueous-ethanol solution in a large container for 3 days with occasional well shaking. The extract was filtered through a Whatman paper and then, dried by a rotary evaporator at 40°C (26) that yielded 60 g of dried hydroalcoholic extract (%12 w/w).
2.4. Total Phenolic Content of Red Lentil
The content of total phenolic compounds in the crude extract was estimated by Folin-Ciocalteu reagent and catechin as a standard in triplicate (27).
Results were calculated in catechin equivalent milligrams per gram of plant seeds.
2.5. Animals
Twenty-four Wistar male rats (weighed 180 - 200 g) were provided by the animal house of Ahvaz Jundishapur University of Medical Sciences. The rats were kept in polypropylene cages and given standard rat chow and drinking water ad libitum. The animals were maintained at a controlled condition of temperature (20 ± 2°C) with a 12:12 hour light/dark cycle. The investigation was performed according to the animal ethics committee guidelines for experimental animals (ethics approval code: 1384.12.5).
2.6. Experimental Design
Rats were randomly divided into 4 groups (6 animals in each group) and treated according to the following protocol:
Group I (control), received normal saline (2 mL/kg/day) for 25 days.
Group II (SA-treated), received normal saline (2 mL/kg/day) for 15 days followed by SA (10 mg/kg/day) for 10 days.
Group III: (RLE supplemented, SA-treated) received RLE (100 mg/kg/day) for 15 days followed by SA (10 mg/kg/day) for 10 days.
Group IV (RLE supplemented, SA-treated) received RLE (200 mg/kg/day) for 15 days followed by SA (10 mg/kg/day) for 10 days.
The dose of SA and RLE was chosen on the basis of previous studies (26, 28, 29).
All of the substances were administrated directly into the stomach of rats by gavage.
2.7. Sample Collection
At the end of the experimental period (the day 26), animals were anaesthetized with diethyl ether and blood samples were collected from the jugular vein. Serum was separated by centrifugation for 10 minutes at 3000 rpm and stored at -20°C until analysis. Then, the animals were sacrificed by decapitation and livers were isolated, washed with saline, and weighed quickly.
A part of liver was dissected and fixed immediately in 10% phosphate buffered formaldehyde for histological studies. For biochemical estimations, the second part of the liver was weighed and homogenized (1/10 w/v) in ice-cold Tris-HCl buffer (0.1 M, pH 7.4).
Protein content in homogenates was measured by the method of Bradford (30), using crystalline BSA as standard.
2.8. Serum Enzyme Analysis
ALT and AST activities were assayed according to Reitman and Frankel (31) using commercially available kits. ALP was estimated according to King (32).
2.9. Sulfhydryl Groups Assay
The levels of sulfhydryl groups (SH-groups) in the tissue were measured following the method described by Ellman (33), based on the formation of a yellow colored complex with the Ellman reagent (DTNB). Homogenates were immediately precipitated with 0.1 mL of 25% TCA and the precipitate was removed after centrifugation. Free endogenous-SH was assayed in a 3-mL glass by adding 2 mL of 0.5 mM DTNB prepared in 0.2 M phosphate buffer (pH = 8) to 0.1 mL of the supernatant and the absorbance was read at 412 nm using a Systronic spectrophotometer.
2.10. Catalase Activity Assay
Catalase (CAT) activity in the tissue was assessed based on the protocol developed by Aebi (34). Accordingly, a 250-µL of 0.066 M H2O2 was added to a cuvette containing 200 µL phosphate buffer, and then, 50 µL of tissue supernatant (obtained after centrifugation of tissue homogenate at 12,000 g for 20 minutes at 4°C) was added and OD decrease was measured at 240 nm for 60 seconds. One unit of activity equals the moles of H2O2 degraded (per minute), divided by the number of milligrams of protein in the tissue supernatant. The molar extinction coefficient of 43.6 M-1 cm-1 was used to determine CAT activity.
2.11. Superoxide Dismutase Activity
Tissue supernatant obtained after centrifugation at 12,000 g for 20 minutes at 4°C was measured spectrophotometrically by calculating the rate of inhibition of auto-oxidation of hematoxylin for the assay of SOD according to the method previously described (35) and expressed as unit/mg protein.
2.12. Lipid Peroxidation Assay
The lipid peroxidation was expressed by measuring the amounts of malondialdehyde (MDA) via the TBA color reaction using the method developed by Buege and Aust (36). Briefly, 0.5 mL of liver homogenate was mixed with 2.5 mL of TCA (10%, w/v), the samples were centrifuged at 3000 rpm for 10 minutes and then, 2 mL of each sample supernatant was transferred to a test tube containing 1 mL of TBA solution (0.67%, w/v). The mixture was kept in boiling water for 10 minutes, forming a pink color solution. The mixture was then cooled immediately and the absorbance was measured at 532 nm by spectrophotometer. The concentration of MDA was calculated based on the absorbance coefficient of the TBA-MDA complex (ε = 1.56 × 105 cm-1 M-1) and expressed as nM/mg protein.
2.13. Statistical Analysis
Data analysis was performed using the Prism 5.0 (San Diego, CA, USA) statistical package program. The Kolmogorov-Smirnov test was used to investigate the normality of the data. Results were expressed as mean ± SD and all statistical comparisons were made by means of the one-way ANOVA test followed by the Tukey post hoc analysis and P values less than 0.05 were considered significant.
3. Results
3.1. Total Phenolic Content of Red Lentil
In the present study, the total phenolic content of RLE was 5% (50.6 mg catechin equivalent/g of seeds dry weight).
3.2. Serum Markers of Liver Damage
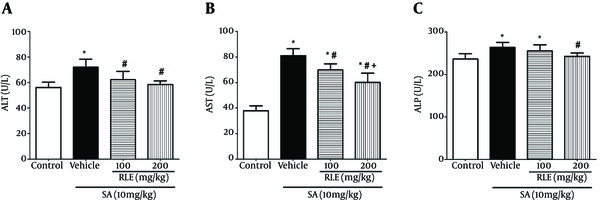
Figure 1 shows the effect of RLE on SA induced changes in activities of liver enzymes in rats.
In the SA-intoxicated group, serum activity of ALT, AST, and ALP increased significantly (P < 0.05) in comparison with those of the control group. Also, treatment with RLE decreased serum activity of ALT, AST, and ALP significantly in contrast with those of the SA-treated group (P < 0.05) except in case of 100 mg/kg RLE in ALP measurement (Figure 1).
Effect of RLE on Serum Liver Enzymes Levels in Experimental Rats Treated with SA
3.3. Oxidative Stress Markers
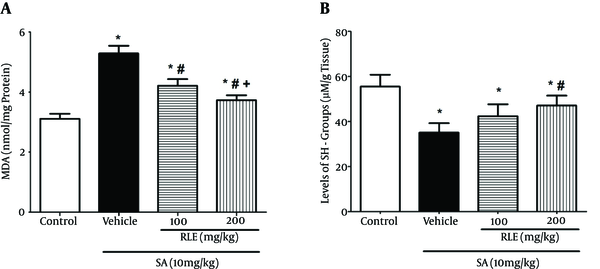
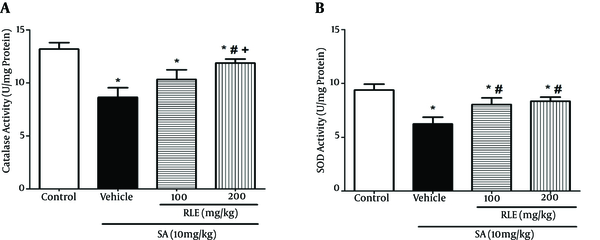
In the SA group, MDA elevated while levels of sulfhydryl groups, CAT, and SOD decreased in comparison with those of the control group (Figures 2 and 3). Also, the current study results showed a significant decrease in SOD and CAT activities of the SA-treated group in contrast with those of the control group, whereas free radicals were generated in this group more than the control group.
Effect of RLE on the Level of MDA and SH-Groups in the Liver of Rats Exposed to SA
Effect of RLE on the Activity of CAT and SOD in Liver of the Rats Treated with SA
MDA decreased in the group treated with red lentil extract that was significantly different from that of the animals exposed to arsenic (P < 0.05).
Comparison of the SA-treated and RLE treated groups revealed increased sulfhydryl groups, CAT, and SOD in a dose-dependent manner in RLE treated groups and the difference was significant only in groups that received 200 mg/kg (P < 0.05). It should be added that the increase of SOD in the group treated with 100 mg/kg RLE was also significant (P < 0.05).
Differences of sulfhydryl groups, SOD, and CAT levels between the groups that received 100 and 200 mg/kg of RLE were insignificant.
3.4. Histopathological Changes in Liver
The histopathological changes in rat liver were examined in H&E stained sections. In the SA-treated group (Figure 4B), severe changes were observed in liver morphology including hepatocytes necrosis, accumulation of inflammatory cells in priportal area, cell swelling in hepatocytes around the central vein compared with those of the control group (Figure 4A).
Photomicrographs of the Liver in the 7 Experimental Groups (H&E); Magnification X400
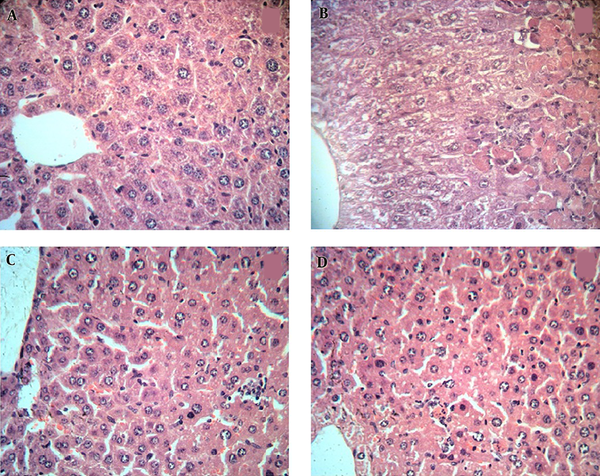
Treatment with RLE improved histological changes including inflammation and necrosis in comparison with the SA-treated group (Figure 4C - 4D).
4. Discussion
Arsenic is a potent and hazardous pollutant that has a complex metabolism pathway and is classified as a potential human carcinogen agent (3). While arsenic compounds are reacting to the molecules in the chain reactions, free radicals are generated and then, cellular oxidative damage start and lead to many biological complications (37). The oxidative stress is induced via multiple mechanisms, especially sodium arsenite-induced ROS, such as superoxide anions and hydroxyl radicals, which exert their effects directly or indirectly on cellular materials (38). Clearly, antioxidants receive great attention in the maintenance of health and chemoprevention of disorders and diseases. Hence, much attention is paid to the antioxidant properties of foodstuff rich in polyphenols and other biologically important metabolites that participate in oxidative processes in the development of degenerative diseases (39).
Lentils are the excellent source of macronutrients such as protein, fatty acids, fibers, and carbohydrates, as well as contain photochemicals, which can be categorized into phenolic acids, flavanols, soyasaponins, phytic acid, and condensed tannins (18).
Lentils showed the highest total antioxidant capacity among tested pulses measured by ferric reducing antioxidant power and total radical-trapping antioxidant parameter measures, but came second to broad beans by Trolox equivalent antioxidant capacity measure. On the other hand, flavonoids such as glycosides of flavonols and flavones are mainly present in the seed coat of lentils (18, 40).
Xu and Chang found that lentils had the highest antioxidant capacity when measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH) in comparison with green pea, yellow pea, and chick pea (41). According to the United States department of agriculture (USDA), oxygen radical absorbing capacity (ORAC) values 2007, lentils had a higher ORAC value than most of the common fruits and vegetables including apples, blackberries, figs, cherries, pears, oranges, garlic, cabbage, and almonds (2). Lentil seeds are used nowadays in the folk medicine of many ethnicities to treat different illnesses. They are used orally to treat diabetes, topically as a water-paste to treat skin infections, and burns, after being roasted, milled, and applied directly to the affected areas (42, 43). Houshmand et al. revealed that hydroalcoholic extract of red lentil had protective effect on catatonia induced by perphenazine in rats (44).
The current study results showed that the administration of SA stimulated lipid peroxidation with simultaneous decrease of antioxidant enzymes activity ameliorated by administration of RLE. Obviously, liver dysfunction is accompanied by elevated levels of hepatic marker enzymes, which are indicative of cellular leak and loss of cell membrane integrity in the liver (45, 46). It is proven that the most commonly used biochemical parameters to detect liver damage are high levels of cytosolic and mitochondrial AST and ALT (47, 48).
During the current investigation, increased serum hepatic enzymes were observed, which was due to exposure to SA. In the current study, hepatic serum marker enzymes of pretreated groups with RLE in comparison with those of the control group were significantly restored that offered protective effect of RLE against SA toxicity in rats. As a result, the protective effect of RLE may be from its active components such as polyphenols with a membrane stabilizing activity (49). On the other hand, Jomova et al. reported that lipid peroxidation was considered a sensitive marker of SA toxicity (50). In addition, El-Demerdash et al. and Flora reported the increased level of MDA in the various tissues of rats treated with SA (51, 52). For this result, in the present study cellular damage was followed by lipid peroxidation, which is one of the main manifestations of oxidative damage and plays an important role in the toxicity of SA (53). Besides the reported studies, the current study results showed a significant decrease in the levels of MDA in rats pretreated with RLE (Figure 2). In other words, presence of polyphenols in red lentil extract, recognized as excellent scavengers of free radicals, could inhibit lipid peroxidation and protein carbonylation (54). Furthermore, GSH, a tripeptide cysteine rich-protein, enters in the maintenance of cytoplasmic and membrane thiol status with antioxidant and powerful nucleophilic activities vital for cellular protection, such as detoxification of ROS.
Sachdeva also showed a significant depletion of hepatic GSH in rats intoxicated by SA (55). It seems that GSH decreases in exposure to SA and is dependent on the consumption of GSH as a scavenger of free radicals or substrate of GPx (56, 57). Whereas hepatic GSH rose in the RLE-treated groups in comparison with the SA-treated group; it is probable that polyphenols found in RLE could play a role similar to that of GSH (58). It should be noted that superoxide dismutase (SOD) and CAT enzymes are involved to defend against the reactive oxygen species by reduction of H2O2 to water and oxygen that protect the cells from the oxidative damage of H2O2 and OH (59). However, the enzymatic (SOD, CAT, and GPx) activity in the RLE-treated groups in comparison with those of the SA-treated groups significantly increased that could indicate a correlation between RLE pretreatment and enzymatic activities. In other words, in critical conditions (exposure to SA) enzymatic activities are interrupted and RLE could change them to semiphysiological conditions. In summary, pretreatment with RLE in rats intoxicated with SA showed a significant recovery of hepatic enzymatic antioxidant systems. In addition, Amarowicz et al. reported the reducing power of red lentil as well (60).
On the other hand, the major bioactive compound found in high concentration in RLE was tannin fraction including polyphenols reported to possess metal chelating property and high antioxidant activity since polyphenols were potent scavengers of superoxide anions, peroxides, as well as hydroxyl and peroxyl radicals (61); this may be the main reason for the anti-hepatotoxic activity of RLE against SA-induced oxidative hepatic dysfunction and results of this study indicated them.
5. Conclusions
In conclusion, the current study results demonstrated that SA was capable of causing marked oxidative stress, depleting the antioxidants and inhibiting the activities of antioxidant enzymes. The treatment with RLE could significantly attenuate the SA-induced oxidative hepatotoxicity and it was observed that its therapeutic potential could be used as a cost-effective safe herbal antioxidative agent to treat SA toxicity.
Acknowledgements
References
-
1.
Balakumar B, Kumaresan S, Venugopal R, Sivasubramanian V. Clastogenic effect of sodium arsenite in experimental rats and ameliorative effects of antioxidant vitamins C and E. Indian J Sci Technol. 2010;3(6):642-7.
-
2.
Amayo KO, Raab A, Krupp EM, Marschall T, Horsfall MJ, Feldmann J. Arsenolipids show different profiles in muscle tissues of four commercial fish species. J Trace Elem Med Biol. 2014;28(2):131-7. [PubMed ID: 24332310]. https://doi.org/10.1016/j.jtemb.2013.11.004.
-
3.
Kulshrestha A, Jarouliya U, Prasad GBKS, Flora SJS, Bisen PS. Arsenic induced abnormalities in glucose metabolism, biochemical basis and potential therapeutic and nutritional interventions. World J Transl Med. 2014;3(2):96. https://doi.org/10.5528/wjtm.v3.i2.96.
-
4.
French VM, Cooper RA, Molan PC. The antibacterial activity of honey against coagulase negative staphylococci. J Antimicrob Chemother. 2005;56(1):228-31. [PubMed ID: 15941774]. https://doi.org/10.1093/jac/dki193.
-
5.
Xie H, Huang S, Martin S, Wise JS. Arsenic is cytotoxic and genotoxic to primary human lung cells. Mutat Res Genet Toxicol Environ Mutagen. 2014;760:33-41. [PubMed ID: 24291234]. https://doi.org/10.1016/j.mrgentox.2013.11.001.
-
6.
Agusa T, Trang PT, Lan VM, Anh DH, Tanabe S, Viet PH, et al. Human exposure to arsenic from drinking water in Vietnam. Sci Total Environ. 2014;488-489:562-9. [PubMed ID: 24262873]. https://doi.org/10.1016/j.scitotenv.2013.10.039.
-
7.
Muhammad A, Odunola OA, Gbadegesin MA, Adegoke AM, Olugbami JO, Uche NS. Modulatory role of acacia honey from north west nigeria on sodium arsenite induced clastogenicity and oxidative stress in male wistar rats. Nat Prod Res. 2015;29(4):321-6. [PubMed ID: 25105348]. https://doi.org/10.1080/14786419.2014.940945.
-
8.
Odunola OA, Akinwumi KA, Ogunbiyi B, Tugbobo O. Interaction and enhancement of the toxic effects of sodium arsenite and lead acetate in wistar rats. Afr J Biomed Res. 2007;10(1). https://doi.org/10.4314/ajbr.v10i1.48972.
-
9.
Sharma A, Sharma MK, Kumar M. Modulatory role of Emblica officinalis fruit extract against arsenic induced oxidative stress in Swiss albino mice. Chem Biol Interact. 2009;180(1):20-30. [PubMed ID: 19428342]. https://doi.org/10.1016/j.cbi.2009.01.012.
-
10.
Aruoma OI, Somanah J, Bourdon E, Rondeau P, Bahorun T. Diabetes as a risk factor to cancer: functional role of fermented papaya preparation as phytonutraceutical adjunct in the treatment of diabetes and cancer. Mutat Res. 2014;768:60-8. [PubMed ID: 24769427]. https://doi.org/10.1016/j.mrfmmm.2014.04.007.
-
11.
Youn UJ, Miklossy G, Chai X, Wongwiwatthananukit S, Toyama O, Songsak T, et al. Bioactive sesquiterpene lactones and other compounds isolated from Vernonia cinerea. Fitoterapia. 2014;93:194-200. [PubMed ID: 24370662]. https://doi.org/10.1016/j.fitote.2013.12.013.
-
12.
Murphy MP. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med. 2014;66:20-3. [PubMed ID: 23603661]. https://doi.org/10.1016/j.freeradbiomed.2013.04.010.
-
13.
Schloss JM, Vitetta L. Antioxidants to abrogate free radicals, new insights to challenge currently held beliefs. Aust J Herb Med. 2014;26(1):4.
-
14.
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113 Suppl 9B:71-88. [PubMed ID: 12566142].
-
15.
Giuseppe DD, Wolk A. Diet and rheumatoid arthritis development, what does the evidence say? Int J Clin Rheumtol. 2014;9(2):169-82. https://doi.org/10.2217/ijr.14.9.
-
16.
Amarowicz R, Troszynska A, Shahidi F. Antioxidant activity of almond seed extract and its fractions. J Food Lipids. 2005;12(4):344-58. https://doi.org/10.1111/j.1745-4522.2005.00029.x.
-
17.
Sabate J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328(9):603-7. [PubMed ID: 8357360]. https://doi.org/10.1056/NEJM199303043280902.
-
18.
Zou Y, Chang SK, Gu Y, Qian SY. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J Agric Food Chem. 2011;59(6):2268-76. [PubMed ID: 21332205]. https://doi.org/10.1021/jf104640k.
-
19.
Amarowicz R, Pegg RB. Legumes as a source of natural antioxidants. Eur J Lipid Sci Technol. 2008;110(10):865-78. https://doi.org/10.1002/ejlt.200800114.
-
20.
Madhujith T, Amarowicz R, Shahidi F. Phenolic antioxidants in beans and their effects on inhibition of radical induced DNA damage. J Am Oil Chem Soc. 2004;81(7):691-6. https://doi.org/10.1007/s11746-004-963-y.
-
21.
Amarowicz R, Troszynska, A. Antioxidant activity of extract of pea and its fractions of low molecular phenolics and tannins. Pol J Food Nutr Sci. 2003;12(53):10-5.
-
22.
Amarowicz R, Troszynska A, Pegg RB. Antioxidative and radical scavenging effects of phenolics from Vicia sativum. Fitoterapia. 2008;79(2):121-2. [PubMed ID: 18178017]. https://doi.org/10.1016/j.fitote.2007.07.018.
-
23.
Van Duyn MA, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. J Am Diet Assoc. 2000;100(12):1511-21. [PubMed ID: 11138444]. https://doi.org/10.1016/S0002-8223(00)00420-X.
-
24.
Kim SK, Wijesekara I. Development and biological activities of marine derived bioactive peptides, a review. J Funct Foods. 2010;2(1):1-9. https://doi.org/10.1016/j.jff.2010.01.003.
-
25.
Zhang Z, Liao L, Moore J, Wu T, Wang Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009;113(1):160-5. https://doi.org/10.1016/j.foodchem.2008.07.061.
-
26.
Rahmani A, Goudarzi M, Rashidi Nooshabadi M, Houshmand G, Khadem Haghighian H. Protective effect of red lentil, (lens culinaris) extract against carbon tetrachloride induced hepatotoxicity in mice. J Babol Univ Med Sci. 2014;16(2):49-55.
-
27.
Amarowicz R, Pegg RB, Rahimi Moghaddam P, Barl B, Weil JA. Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84(4):551-62. https://doi.org/10.1016/s0308-8146(03)00278-4.
-
28.
Kadirvel R, Sundaram K, Mani S, Samuel S, Elango N, Panneerselvam C. Supplementation of ascorbic acid and alpha-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats. Hum Exp Toxicol. 2007;26(12):939-46. [PubMed ID: 18375637]. https://doi.org/10.1177/0960327107087909.
-
29.
Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M. The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull. 2001;55(2):301-8. [PubMed ID: 11470331].
-
30.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248-54. [PubMed ID: 942051].
-
31.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56-63. [PubMed ID: 13458125].
-
32.
King J. The phosphohydrolases and alkaline phosphatases, practical clinical enzymology. London: Van Nostrand Company; 1965.
-
33.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-7. [PubMed ID: 13650640].
-
34.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-6. [PubMed ID: 6727660].
-
35.
Martin JJ, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987;255(2):329-36. [PubMed ID: 3036004].
-
36.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10. [PubMed ID: 672633].
-
37.
Tokar EJ, Kojima C, Waalkes MP. Methylarsonous acid causes oxidative DNA damage in cells independent of the ability to biomethylate inorganic arsenic. Arch Toxicol. 2014;88(2):249-61. [PubMed ID: 24091636]. https://doi.org/10.1007/s00204-013-1141-2.
-
38.
Bodhank SL, Adil M, Visnagri A, Kumar VS, Kandhar AD, Ghosh P. Protective effect of naringin on sodium arsenite induced testicular toxicity via modulation of biochemical perturbations in experimental rats. Pharmacologia. 2014;5(6):222-34. https://doi.org/10.5567/pharmacologia.2014.222.234.
-
39.
Goyal S, Deepak K, Reddy P, Pradeep K, Swapna LA. Antioxidants and their implication in oral health and general health. Int J Case Rep Imag. 2014;5(4):258-63. https://doi.org/10.5348/ijcri-201555-RA-10010.
-
40.
Amarowicz R, Estrella I, Hernandez T, Duenas M, Troszynska A, Agnieszka K, et al. Antioxidant activity of a red lentil extract and its fractions. Int J Mol Sci. 2009;10(12):5513-27. [PubMed ID: 20054484]. https://doi.org/10.3390/ijms10125513.
-
41.
Xu BJ, Chang SK. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72(2):S159-66. [PubMed ID: 17995858]. https://doi.org/10.1111/j.1750-3841.2006.00260.x.
-
42.
Deshpande SS. Food legumes in human nutrition: a personal perspective. Crit Rev Food Sci Nutr. 1992;32(4):333-63. [PubMed ID: 1297325]. https://doi.org/10.1080/10408399209527603.
-
43.
Faris MAIE, Takruri HR, Issa AY. Role of lentils, (Lens culinaris L.) in human health and nutrition, a review. Med J Nutrition Metab. 2013;6(1):3-16. https://doi.org/10.1007/s12349-012-0109-8.
-
44.
Houshmand G, Tarahomi S, Arzi A, Goudarzi M, Bahadoram M, Rashidi-Nooshabadi M. Red Lentil Extract: Neuroprotective Effects on Perphenazine Induced Catatonia in Rats. J Clin Diagn Res. 2016;10(6):5-8. [PubMed ID: 27504309]. https://doi.org/10.7860/JCDR/2016/17813.7977.
-
45.
Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2005;25(1):193-7. [PubMed ID: 15499043]. https://doi.org/10.1161/01.ATV.0000148324.63685.6a.
-
46.
Aghel N, Kalantari H, Rezazadeh S. Hepatoprotective effect of Ficus carica leaf extract on mice intoxicated with carbon tetrachloride. Iran J Pharm Res. 2011;10(1):63-8. [PubMed ID: 24363682].
-
47.
Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007;45(9):1551-7. [PubMed ID: 17658209]. https://doi.org/10.1016/j.fct.2007.06.007.
-
48.
Kalantari H, Aghel N, Bayati M. Hepatoprotective effect of Morus alba L. in carbon tetrachloride induced hepatotoxicity in mice. Saudi Pharmaceut J. 2009;17(1):90-4.
-
49.
Omale J, Okafor PN. Comparative antioxidant capacity, membrane stabilization, polyphenol composition and cytotoxicity of the leaf and stem of Cissus multistriata. African J Biotechnol. 2008;7(17).
-
50.
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95-107. [PubMed ID: 21321970]. https://doi.org/10.1002/jat.1649.
-
51.
El-Demerdash FM, Yousef MI, Radwan FM. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009;47(1):249-54. [PubMed ID: 19049818]. https://doi.org/10.1016/j.fct.2008.11.013.
-
52.
Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008;128(4):501-23. [PubMed ID: 19106443].
-
53.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329-54. [PubMed ID: 24692350]. https://doi.org/10.1152/physrev.00040.2012.
-
54.
Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872-9. [PubMed ID: 12361782].
-
55.
Sachdeva S, Flora SJ. Efficacy of some antioxidants supplementation in reducing oxidative stress post sodium tungstate exposure in male wistar rats. J Trace Elem Med Biol. 2014;28(2):233-9. [PubMed ID: 24613855]. https://doi.org/10.1016/j.jtemb.2014.01.004.
-
56.
Aquilano K, Baldelli S, Ciriolo MR. Glutathione, new roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196. [PubMed ID: 25206336]. https://doi.org/10.3389/fphar.2014.00196.
-
57.
Feng S, Deng H; Zheng X; Wang D; Gong Y; Wang Q. Systematic analysis of reactivities and fragmentation of glutathione and its isomer Glucysgly. J Phys Chem A. 2014;118(37):8222-8. [PubMed ID: 24734962]. https://doi.org/10.1021/jp501015k.
-
58.
Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56(11):317-33. [PubMed ID: 9838798].
-
59.
Shen DS, Tao XQ, Shen CC, Shentu JL, Wang MZ. Antioxidant defense enzymes response following polychlorinated biphenyls exposure to eisenia fetida in actual polluted soil. Adv Mater Res. 2014;1010:142-6. https://doi.org/10.4028/www.scientific.net/AMR.1010-1012.142.
-
60.
Amarowicz R, Estrella I, Hernandez T, Robredo S, Troszynska A, Kosinska A, et al. Free radical scavenging capacity, antioxidant activity, and phenolic composition of green lentil, (Lens culinaris). Food Chem. 2010;121(3):705-11. https://doi.org/10.1016/j.foodchem.2010.01.009.
-
61.
Denev P, Kratchanova M, Ciz M, Lojek A, Vasicek O, Nedelcheva P, et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014;157:37-44. [PubMed ID: 24679749]. https://doi.org/10.1016/j.foodchem.2014.02.022.