Abstract
Background:
Crocus sativus L., (Iridaceace), known as saffron, is used in traditional medicine for different purposes such as memory improvment. Crocin is one of the pharmacologically active constituents responsible for many medicinal properties of saffron.Objectives:
In this study, the effects of crocin, an active saffron constituent, on hyoscine induced memory impairment and its effect on protein and mRNA transcript levels of CREB (cAMP response element binding protein), BDNF (brain-derived neurotrophic factor), and phospho-CREB (p-CREB) were investigated in rat hippocampus.Methods:
Rats were intraperitoneally treated with crocin (10, 20, and 40 mg/kg), normal saline (1 mL/kg), and rivastigmine (1.5 mg/kg) 30 minutes prior to hyoscine (1 mg/kg) for five days. The effects were studied on spacial learning and memory using Morris water maze. Protein and transcript levels of CREB and BDNF were analyzed using western blotting and qRT PCR methods, respectively.Results:
Five days treatment with crocin (20 and 40 mg/kg) and rivastigmine significantly increased the presence of animals in target quadrant in probe trial on the 8th day compared to hyoscine. Crocin (20 mg/Kg) increased significantly BDNF and CREB protein and transcript levels compared to hyoscine.Conclusions:
Crocin improved memory impairment induced by hyoscine through reduction of the latency to find the platform in the probe trial in Morris water maze test. Crocin improved memory probably due to the increase of BDNF and CREB protein and transcript levels.Keywords
Crocin BDNF CREB p-CREB Morris Water Maze Test Spatial Memory
1. Background
Learning is a process in which we can access information about the world, and memory is a mechanism for encoding, storing, and recalling of stored information. Currently, the hypothesis suggests that new memories are encoded as changes in synaptic efficacy induced by synaptic activity, and maintenance of these changes requires protein synthesis. Brain processes information and stores memory via changes in synaptic connections between neurons or in synaptic plasticity (1). Physiological changes in the intensity of the message are often associated with synaptic structural changes and since the memories are stored in the brain synapses, it seems that synaptic connections have an important role in cellular mechanisms of learning and memory (1, 2). Hippocampus is well known to be involved in learning and memory (3).
Strong evidence shows that learning triggers activation of NMDA, AMPA, and metabotropic glutamate receptors and then a sequence of events in cellular signaling that are responsible for learning and synaptic plasticity occures. Protein kinase signaling cascades are activated downstream of glutamate receptors, including calcium-calmodulin-dependent protein kinase II (CaMKII), phospholipase C (PLC)/protein kinase C (PKC), cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP response element binding protein (CREB), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated protein kinase (ERK), and phosphatidylinositol 3-kinase (PI3K) pathways. The activation of these factors leads to the synthesis of new proteins and expression of some genes (Arc and C-fos), which ultimately changes gene expression and improves the structural growth of synapses and new synaptic connections (4).
Unfortunately, increased life expectancy in the world is associated with increased cognitive disorders consisting of memory loss and reduced ability to form long-term memories. Age-related memory impairment is a common condition characterized by symptoms of mild cognitive impairment (3). Memory and learning impairment due to the neurodegenerative diseases such as Alzheimer’s disease (AD) would be a concern (5). This disorder interferes with normal functions of the patients’ life. Therefore, the discovery and improvement of strategies that can restore memory and prevent memory disturbance seem to be intransitive (5). AD is the most prevalent type of dementia in seniors. Currently, there is no efficient treatment strategy for this disease. AD is characterized by accumulation of β-amyloid called “senile plaques”, unusual phosphorylated and aggregated microtubule-associated protein tau, synaptic functional impairment, and finally neuronal death (6). Molecular mechanisms of Alzheimer’s disease are currently unknown although evidence suggests that multiple intracellular pathways and signal transduction cascades are involved in pathophysiology and treatment of AD and depression (4). Many anti-Alzheimer’s drugs increase monoamine levels in acute usage, but the need for chronic treatment leads to a hypothesis that explains the necessity of long-term accommodation for chronic treatment that may cause changes in plasticity, neurogenesis, and synaptogenesis. The role of CREB and BDNF (brain-derived neurotrophic factor) is clear in these changes (4).
Crocus sativus L., known as Saffron, is an herbaceous stemless plant of the family Iridaceae, which is widely cultivated in Spain, France, Greece, and the eastern regions of Iran (5, 7). Crocin is the glycosylated ester of crocetin, which is a water-soluble carotenoid responsible for the color and many of the pharmacological properties of saffron (8, 9). Several biological activities have been attributed to either saffron or its active constituents such as antioxidant (10, 11), anxiolytics and antidepressants (12, 13), antinociceptive and anti-inflammatory (14), antiseizure (15, 16), antitussive (17), reduction of withdrawal syndrome (18), improvement of memory and learning (19), hypotensive (20, 21), anticancer (22), and antigenotoxic (23) effects. Although there is no evidence of crocin passage from the blood brain barrier, according to studies crocetin has the ability to pass this pharmacological barrier. In addition, concerning the central effects of crocin, it seems that crocin is decomposed to crocetin and its attached sugar in blood and crocetin is the reason for crocin biological effects in the brain (24).
In literature there are many studies investigating the role of crocin in learning and memory. For example, administration of aqueous extract of saffron improved ethanol-induced memory impairment due to the interaction with NMDA receptors (25). Another study showed that saffron and its active constitutes, crocin and safranal, could improve the impaired memory induced by hyoscine (26). Previousely we showed that antidepressant effects of saffron and crocin in long term administration could be related to the effect on the expression of some proteins that are involved in depressive disorders including CREB and BDNF (27, 28).
2. Objectives
Regarding the role of these proteins in memory and learing, in this study, the effects of crocin on learning and memory as well as its effect on the levels of CREB and BDNF were investigated. For this purpose, hyoscine was used for memory impairment in animal model. Based on our knowledge, it is the first to investigate the effects of crocin on the expression of proteins involving in memory.
3. Methods
3.1. Animals
Adult male Wistar Albino rats, weighing 230 ± 30 g, were provided by Animal house, school of pharmacy, Mashhad University of Medical Sciences, Iran. The rats were maintained under 12-h light/dark cycle, 22 ± 2°C, and 40% - 50% humidity conditions in the colony room. The animals had free access to food and water before and during the study. This study was approved by the ethics committee (No. 910450) of Mashhad University of Medical Sciences.
The rats were randomly divided into 6 groups of 6 rats (n = 6):
1) Control (normal saline); 2) hyoscine 1 mg/Kg/day; 3) rivastigmine 1.5 mg/kg/day plus hyoscine; 4) crocin 10 mg/kg/day plus hyoscine; 5) crocin 20 mg/kg/day plus hyoscine; and 6) crocin 40 mg/kg/day plus hyoscine. Rivastigmine and crocin were administrated 30 minutes before hyoscine. The rats received treatments intraperitoneally (IP.) for 5 days.
3.2. Chemicals
High Pure RNA Tissue Kit (#12033674001, Roche, Germany) for RNA extraction, EXPRESS One-Step SYBR® GreenER™ SuperMix Kit (#11780-200, Invitrogen, USA) for qRT-PCR, and Bradford Protein Assay Kit (#500-0006, BIO-RAD) to determine protein contents were used. Rivastigmine was obtained from Cipla, India. Rabbit monoclonal anti-serum against CREB (#9197, Cell Signaling, USA), mouse monoclonal anti-serum against p-CREB (Ser133) (#9196, Cell Signaling, USA), rabbit polyclonal antiserum against BDNF (#ab46176, Abcam, USA), mouse and rabbit monoclonal anti-serums against β-actin (# 3700 and # 4970, Cell Signaling, USA), and anti-mouse and rabbit horse radish peroxidase labeled IgG (#7076 and #7074, Cell Signaling, USA) were used in western blot. Primers were purchased from Metabion international AG, Germany (Table 1). Other chemicals were obtained from Sigma-Aldrich, Germany.
Primers Used for qRT-PCR
| Gene | Amplicon Length, bp | |
|---|---|---|
| Β-actin | Forward 5′-GGGAAATCGTGCGTGACATT-3′ | 76 |
| Reverse 5′- GCGGCAGTGGCCATCTC-3′ | ||
| CREB | Forward 5′-CCAAACTAGCAGTGGGCAGT-3′ | 140 |
| Reverse 5′- GAATGGTAGTACCCGGCTGA-3′ | ||
| BDNF | Forward 5′-TCTACGAGACCAAGTGTAATCC-3′ | 152 |
| Reverse 5′- TATGAACCGCCAGCCAAT-3′ |
3.3. Crocin Extraction
Crocin was extracted and purified in a way previously described by Hadizadeh et al. (29). 10 g of saffron stigma powders were suspended in 25 mL ethanol 80% (0°C) and vortexed for 2 minutes. The suspension was centrifuged (4,000 rpm for 10 minutes) and the supernatant was separated. This step was done 6 times repeatedly by addition of 25 mL ethanol 80% and the resulting extract was kept in a sealed thick walled glass container at -5°C in darkness for 24 days. Washing of the formed crystals was done with acetone to remove remaining water after separation from the solution. The obtained crystals were then dissolved in 120 mL ethanol 80% and kept at -5°C for 20 days. The purity of total crocin was more than 97%, and 10% crocin was obtained from the initial stigma powders. The purity of crocin crystals was determined by UV-visible spectrophotometery and HPLC.
3.4. Morris Water Maze
The effect of ingredients on spatial memory and learning was investigated by Morris water maze test. Morris water maze consisted of a circular water tank (136 cm diameter and 60 cm height). The tank was filled with water (20 ± 10C) to a depth of 35 cm. The maze was located in a room containing extra-maze cues. To conceal the platform, the walls of the pool and the platform were dyed black. The maze was geographically divided into four quadrants (northeast (NE), northwest (NW), southeast (SE), southwest (SW)) and four starting positions (north (N), south (S), east (E), west (W)) that were equally spaced around the perimeter of the pool. A platform (10 cm2) was located one centimeter below the surface of the water in the center of the target quadrant (SE quadrant). A video camera recorded the rats’ swim paths. A tracking system was used to measure traveled distance and swimming speed of rats and the escape latency time. The percentage of time in the target quadrant was also calculated (19).
Rats were given four training trials each day during five days conducted 30 minutes after the last injection. In each training trial, the rats were placed in the water facing the pool wall at one of the four starting positions (north, south, east, or west pole) in different orders for different days and allowed to swim until they reached the platform placed in the target quadrant of the maze in every trial. The latency to find the platform was recorded for up to 60 seconds. The rats were allowed to stay on the platform for 20 seconds. The animals that were unable to locate the hidden platform within 60 seconds were guided to it and allowed to spend at least 20 seconds on it. Escape latency time to locate platform was noted as an index of acquisition (19). After the last day of acquisition, the animals were allowed to rest for 2 days and then on the third day, the platform was removed (on the 8th day of study) and the final trial test was conducted to evaluate the memory of the correct platform location. After the trials, the rats were dried with a towel and placed under a heating lamp before they were returned to the home cage (19, 29).
3.5. Western Blotting
At the end of the experiment (after evaluation of the times spent on swimming in the target zone for different groups on the 8th day of study), the animals were sacrificed by decapitation and the brains were removed and dissected on ice. Each brain was cut in half with a midline incision and the midbrain was removed. Hippocampus was isolated, washed by saline, and rapidly frozen in liquid nitrogen. Tissues were stored at −80°C for subsequent processes. Then, the tissues were homogenized in the homogenization buffer containing Tris-HCl 50 mM (pH: 7.4), 2 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 10 mM β-glycerol phosphate, 0.2% w/v NaDC, 1 mM PMSF, and complete protease inhibitor cocktail using polytron homogenizer (POLYTRON® PT 10 - 35, Kinematica, Switzerland) in ice. Centrifugation was done at 10,000 × g for 15 minutes at 4°C and supernatants were collected on ice. Protein contents were determined using Bradford Protein Assay Kit. Immunoblotting analysis was performed on the prepared samples to evaluate the levels of CREB, p-CREB, and BDNF. Samples containing the same amounts of total protein were loaded on SDS-PAGE gel and then transferred to PVDF membrane using electrophoresis. Blots were blocked using 5% non-fat dry milk in TBST for 3 hours at room temperature. After blocking, the blots were probed with specific primary antibodies at 1:1000 dilutions for 2 hours at room temperature. Membranes were washed 3 times with TBST. Next, the blots were incubated with secondary antibodies at 1:3000 dilutions for 1 hour at room temperature. Finally, an enhanced chemiluminescence reagent (Pierce ECL western blotting substrate) and Alliance Gel-doc (Alliance 4.7 Gel doc, UVtec UK) were used to visualize protein bands. UV Tec software (UK) was used to semi-quantify protein bands intensities, and blots were normalized against intensities of corresponding β-actin protein bands.
3.6. Quantitative RT-PCR
High Pure RNA Tissue Kit was used to extract total RNAs from rat hippocampus according to the manufacturer’s instructions. Then, the quantity and quality of the extracted RNAs were assessed using NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, USA).
qRT-PCR was performed to analyze transcription level of CREB and BDNF using EXPRESS One-Step SYBR® GreenER™ SuperMix Kit for one-step qRT-PCR (according to the manufacturer’s instructions) and a StepOne™ Real-Time PCR System (ABI, USA). Results were analyzed by ΔΔCt method. Real-time PCR was used for all genes according to this protocol: activation of reverse transcriptase and cDNA synthesis (5 minutes @ 50°C), PCR activation (2 minutes @ 95°C), 40 cycles of denaturation (15 seconds @ 95°C), and annealing/ extension (1 minute @ 60°C). Following PCR, melting curve analysis was done by gradually increasing the temperature from 60 to 95°C with a heating rate of 0.3°C/s. Primers for the chosen genes were designed by Beacon designer 7.8 (Biosoft, USA) and their specificity was approved by BLAST (http://www.ncbi.nlm.nih.gov/ tools/primer-blast/). β-actin was applied as endogenous control gene.
3.7. Statistical Analysis
Data are expressed as mean ± SEM. One way and two way ANOVA followed by Tukey-Kramer and Bonferroni post hoc tests were performed to compare means. P values less than 0.05 were considered significant.
4. Results
4.1. Morris Water Maze Test
4.1.1. Effect of Crocin on Time Spent in Target Quadrant on the 8th Day
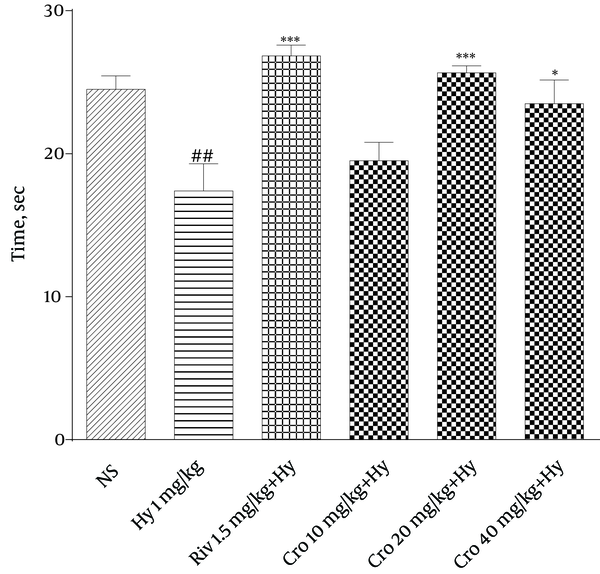
According to Figure 1, hyoscine decreased time spent in target quadrant on the 8th day when compared to the control group (P < 0.01). Rivastigmine and crocin (20 and 40 mg/Kg) increased time spent in target quadrant on the 8th day compared to hyoscine (P < 0.001 and P < 0.05, respectively).
Effect of Crocin on Time Spent in Target Quadrant on the 8th Day in Morris Water Maze Test
4.1.2. Effect of Crocin on Swim Speed in the Target Quadrant and Total Swim Speed on the 8th Day
Results of swim speed in the target quadrant as well as total swim speed on the 8th day showed that there is no significant difference between different groups (data not shown).
4.1.3. Effect of Crocin on Latency Time to Find Platform During Five Days
Two-way ANOVA followed by Bonferroni test [treatment effect: F (5, 137) = 68.63, P < 0.0001, number of days effect (4, 137) = 34.12, P < 0.0001, treatment × number of days interaction: F (20, 137) = 2.209, P = 0.0040] indicated that hyoscine increased latency time to find platform during five days compared to the control group. Rivastigmine and crocin (10, 20, and 40 mg/Kg) decreased latency time to find platform during five days (Figure 2).
Effect of Crocin on Latency Time to Find Platform During Five Days in Morris Water Maze Test

4.1.4. Effect of Crocin on Swim Speed During Five Days
Results of swim speed during five days using two-way ANOVA followed by Bonferroni test [treatment effect: F (5, 160) = 4.434, P = 0.0008, number of days effect (4, 160) = 2.396, P = 0.0526, treatment × number of days interaction: F (20,160) = 0.8282, P = 0.6767] showed no significant difference between different groups (Figure 3).
Effect of Crocin on Swim Speed During Five Days in Morris Water Maze Test
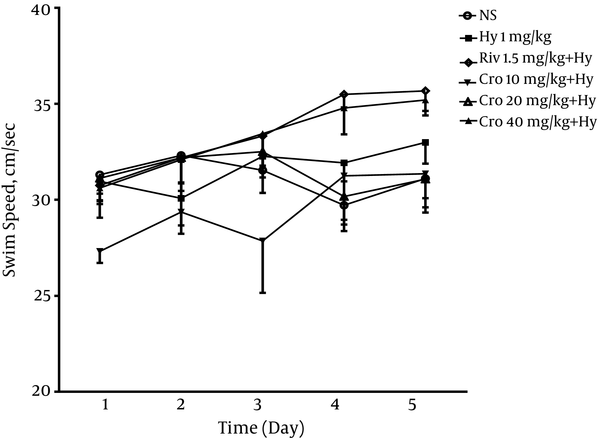
4.2. Western Blot Analysis
4.2.1. Effect of Crocin on the Protein Level of BDNF in Rat Hippocampus
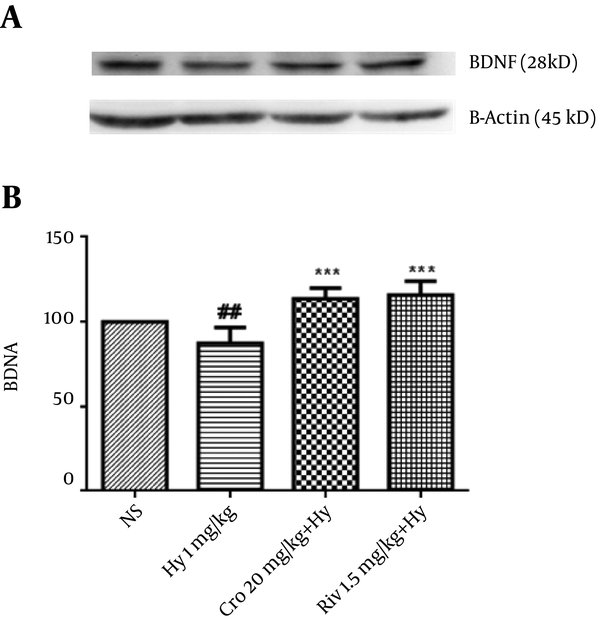
According to Figure 4, hyoscine decreased BDNF protein level compared to the control group (P < 0.01). Crocin (20 mg/kg) significantly increased protein level of BDNF compared to the hyoscine group (P < 0.001). A similar effect was also observed in the rivastigmine group (P < 0.001).
The Effect of Crocin 20 mg/kg on BDNF Level in Rat Hippocampus by Western Blotting
4.2.2. Effect of Crocin on the Protein Level of CREB in Rat Hippocampus
Hyoscine decreased CREB protein level compared to the control group (P < 0.05). Crocin (20 mg/kg) and rivastigmine significantly increased the protein level of BDNF compared to the hyoscine group (P < 0.001) (Figure 5).
The Effect of Crocin (20 mg/kg) on the CREB Protein Level in The Rat Hippocampus by Western Blotting

4.2.3. Effect of Crocin on the Protein Level of p- CREB in Rat Hippocampus
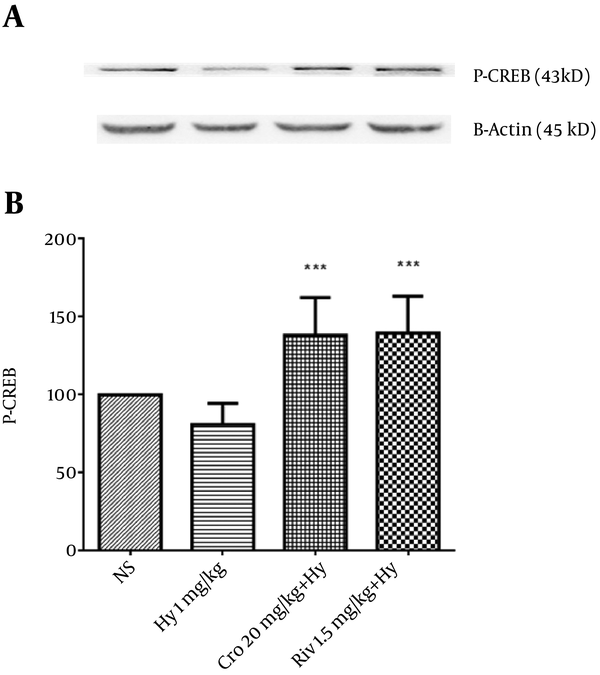
Although hyoscine decreased p-CREB (activated form of CREB) protein level compared to the control group, this effect was not statistically significant. Crocin (20 mg/kg) and rivastigmine significantly increased protein levels of p-CREB compared to the hyoscine group (P < 0.001) (Figure 6).
Effect Crocin (20 mg/kg) on the p-CREB Level in the Rat Hippocampus by Western Blotting
4.3. qRT PCR
4.3.1. Effect of Crocin on the mRNA Level of BDNF in Rat Hippocampus
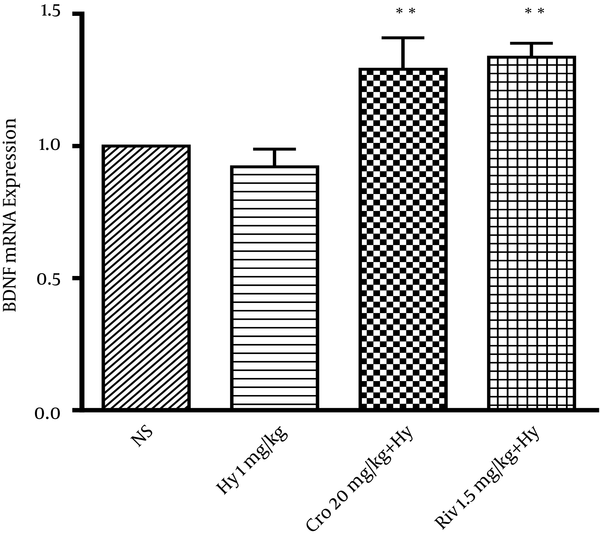
Crocin (20 mg/kg) and rivastigmine significantly increased BDNF transcription level in rat hippocampus (**P < 0.01) compared to the hyoscine group (Figure 7).
Effects of Crocin (20 bmg/Kg) on Transcript Levels of BDNF
4.3.2. Effect of Crocin on the mRNA Level of CREB in Rat Hippocampus
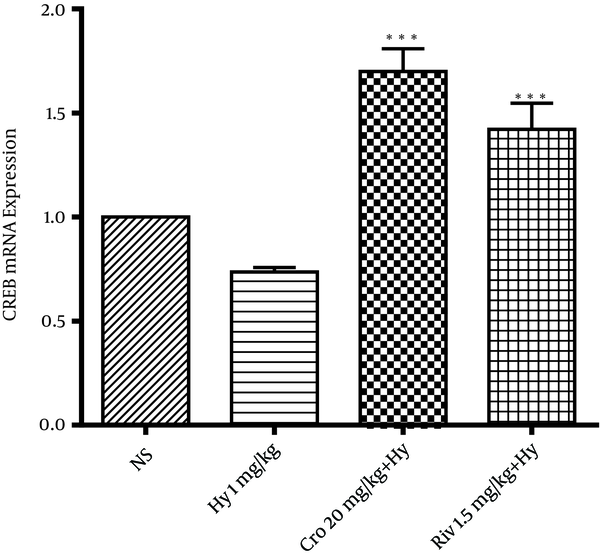
According to Figure 8, crocin (20 mg/kg) and rivastigmine significantly increased CREB transcription level in rat hippocampus (**P < 0.01) compared to the hyoscine group.
Effects of Crocin (20 mg/Kg) on Transcript Levels of CREB
5. Discussion
Memory and learning impairment due to the neurodegenerative diseases such as Alzheimer’s disease would be a concern. This disorder interferes with normal functions of the patients’ life. Therefore, the discovery and improvement of strategies that can restore memory and prevent memory disturbance seem to be intransitive (5). Morris water maze is a good animal model to predict the effects of medicine on memory and learning. Compounds that increase time spent in the target quadrant in probe trial have memory enhancement effects (30). It is believed that memories are stored at the synapses of the brain; accordingly, synaptic plasticity is thought to be an important cellular mechanism for learning and memory. The most studied form of synaptic plasticity in hippocampus is long-term potentiation (LTP) (1). Scopolamine (hyoscine) is an anticholinergic drug that causes dementia by interfering with LTP. It can be used to induce memory impairment in animal models (31). It has been reported that scopolamine causes memory impairment in Morris water maze test through down regulation of protein kinase C and iNOS (32).
In this study, to induce memory impairment, scopolamine and to predict antiamnesic effects of crocin, Morris water maze test were used. Results showed that administration of crocin for 5 days significantly increased the time spent in target quadrant in probe trial compared to the hyoscine group. It has been proven that saffron and its constituents improve memory and learning impairment. For example, it has been suggested that administration of aqueous extract of saffron improves ethanol-induced memory impairment due to the interaction with NMDA receptors (25). Research has shown that aqueous extract of saffron, crocin, and safranal had no effect on intact memory but they could improve the impaired memory induced by hyoscine (26). Another study indicated that saffron and its active constituent crocin could alleviate chronic stress induced learning and memory impairment as well as the oxidative stress damage to the hippocampus (33). Moreover, a study by Hoseinzadeh et al. (2012) suggested that saffron extract and crocin could improve spatial cognitive impairment following chronic cerebral hypoperfusion and these effects may be attributed to their antioxidant effects (19). In addition, in another study, inhibition of LTP induced by acetaldehyde was prevented by ethanolic extract of saffron (34).
To understand the molecular mechanisms involved in antiamnesic effects of crocin, the protein and transcript levels of BDNF, CREB, and p-CREB in rat hippocampus were evaluated by western blotting and qRT-PCR.
Several studies have noted the important role of intracellular pathways regulating neuroplasticity and neurodegeneration in the etiology of memory disorders. According to these studies, AD is the result of neuronal atrophy and decreased neurogenesis, and anti-Alzheimer drugs increase CREB protein and expression of neurotrophic factors (such as BDNF) by stimulating intracellular pathways (4).
BDNF is a neuroprotective dimer protein belonging to neurotrophins that is widely expressed in mammalian brain. BDNF has an important role in the development and maintenance of central and peripheral nervous system, neuronal survival, and their proliferation. This protein acts through two distinct receptors: tropomyosin related kinases (TrKs), which are specific for neurotrophins, and p75NTR that binds to all neurotrophins. These receptors are activated by dimerization and autophosphorylation of tyrosine kinases that induce intracellular signaling pathways and ultimately lead to cell proliferation and neuronal systems protection and survival (35).
BDNF and TrkB are widely distributed in hippocampus and frontal areas of adult brain and BDNF secreting vesicles are available in dendrites and terminal axons of glutamatergic neurons. There is strong evidence on BDNF involvement in long-term memory, so after learning spatial tasks, such as the Morris water maze, an increase in BDNF mRNA level would be observed. On the contrary, blocking BDNF synthesis or blocking TrkB access to BDNF resulted in long-term memory formation impairment. However, silencing of BDNF mRNA by infusion of antisense oligonucleotides or BDNF antibodies before training inhibits the acquisition and learning (1).
Stress and depression reduce the mRNA level of BDNF in hippocampus. Decreased levels of BDNF in the atrophy of nervous cells have been observed. A series of studies in humans have shown that Alzheimer’s disease, schizophrenia, bipolar, and manic disorders reduce BDNF level in plasma while electric shock or antidepressants increase the levels of this protein in serum (36).
Our results showed that crocin (20 mg/kg) could significantly increase both protein and mRNA levels of BDNF and CREB in rat hippocampus as the same as rivastigmine (an anti-Alzheimer drug, positive control) compared to hyoscine. Crocin could also significantly increase the protein level of p-CREB in rat hippocampus. Our results are in agreement with the findings of other studies showing the antidepressant effects of saffron aqueous extract as well as showing that crocin could be attributed to the increase in the protein and mRNA levels of BDNF, CREB, and p-CREB in rat hippocampus following 21 days treatments (27, 28).
The critical role of CREB in neuronal survival and plasticity has been also identified (37). It is suggested that actions of neurotransmitters and neurotrophic factors on adult neurogenesis could be regulated by cAMP-CREB cascade (38). The physiological modulation of neuronal excitability by CREB can affect learning and memory processes through at least three non-exclusive mechanisms. Increasing excitability following over activation of CREB and suppression of after hyperpolarization (AHP) can lead to a threshold decline in the induction of long-term potentiation (LTP) in sensitized neuron. Therefore, a longer-lasting potentiation (late LTP or L-LTP) would occur instead of short-lasting synaptic potentiation (early LTP or E-LTP). On the other hand, CREB inhibition increased the threshold of LTP induction (39).
Conversely, preclinical and clinical studies demonstrate that impaired phosphorylation of CREB may be involved in pathology of neurodegenerative disorders, especially in AD. Accordingly, pharmacological-induced CREB phosphorylation in cortex and hippocampus may indicate new approaches for the development of AD therapeutics.
It is established that CREB can activate the production of BDNF. Not only CREB activates the BDNF production, but also BDNF increases the phosphorylation of CREB. Although many studies have shown the necessity of CREB-mediated transcription for long-term memory stabilization, the mechanisms by which CREB facilitates memory are not fully known. BDNF gene expression induced by CREB is considered as an important mechanistic constituent of CREB memory enhancing effects. Similar to CREB, different studies have demonstrated that BDNF exerts precognitive effects because of its neurotrophic effects resulting in neurite outgrowth and improved synaptogenesis related to neuroplasticity. Therefore, BDNF has been shown to improve learning in both long-term and short-term memory models. Moreover, short-term memory enhancement created by BDNF can be related to up regulation of CREB activity. Studies in CREB null mutant knockout mice showed a regulatory role for CREB in short-term memory through BDNF expression regulation. Regarding all these, in addition to its effects on long-term memory formation, CREB indirectly enhances short-term memory (40).
In summary, this study showed that five days administration of crocin has memory enhancement effects in rat partially due to the increase in protein and transcript levels of CREB and BDNF in hippocampus.
Acknowledgements
References
-
1.
Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89(3):312-23. [PubMed ID: 17942328]. https://doi.org/10.1016/j.nlm.2007.08.018.
-
2.
Haghpanah T, Esmailpour Bezanjani K, Khaki A, Reza M, Sheibani V, Abbasnejad M, et al. Effect of intra-hippocampal injection of Origanum vulgare L. ssp. viridis leaf extract on spatial learning and memory consolidation. KAUMS J. 2011;14(4):380-7.
-
3.
Alberini CM, Chen DY. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35(5):274-83.
-
4.
Roesler R, Schroder N. Cognitive enhancers: focus on modulatory signaling influencing memory consolidation. Pharmacol Biochem Behav. 2011;99(2):155-63. [PubMed ID: 21236291]. https://doi.org/10.1016/j.pbb.2010.12.028.
-
5.
Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65-80.
-
6.
Selkoe DJ. Alzheimer's disease. Cold Spring Harb Perspect Biol. 2011;3(7). [PubMed ID: 21576255]. https://doi.org/10.1101/cshperspect.a004457.
-
7.
Hosseinzadeh H. Saffron: a herbal medicine of third millennium. Jundishapur J Nat Pharm Prod. 2014;9(1):1-2. [PubMed ID: 24644431].
-
8.
Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27(4):475-83. [PubMed ID: 22815242]. https://doi.org/10.1002/ptr.4784.
-
9.
Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4(2):83-6. [PubMed ID: 8347278].
-
10.
Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents, crocin and safranal. Pharmacognosy Magazine. 2009;5(20):419.
-
11.
Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8(3):387-93. [PubMed ID: 16401388].
-
12.
Hosseinzadeh H, Noraei N. Anxiolytic and Hypnotic Effect of Crocus sativus aqueous extract and its constituents, crocin and saftanal, in mice. Phytother Res. 2009;26:768-74.
-
13.
Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant Effect of Crocus Sativus L. Stigma Extracts and Their Constituents, Crocin and Safranal, in Mice. Acta Horticulturae. 2004;(650):435-45. https://doi.org/10.17660/ActaHortic.2004.650.54.
-
14.
Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. [PubMed ID: 11914135].
-
15.
Hosseinzadeh H, Sadeghnia HR. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomedicine. 2007;14(4):256-62. [PubMed ID: 16707256]. https://doi.org/10.1016/j.phymed.2006.03.007.
-
16.
Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76(7-8):722-4. [PubMed ID: 16253437]. https://doi.org/10.1016/j.fitote.2005.07.008.
-
17.
Hosseinzadeh H, Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia. 2006;77(6):446-8. [PubMed ID: 16814486]. https://doi.org/10.1016/j.fitote.2006.04.012.
-
18.
Hosseinzadeh H, Jahanian Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother Res. 2010;24(5):726-30. [PubMed ID: 19827024]. https://doi.org/10.1002/ptr.3011.
-
19.
Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26(3):381-6. [PubMed ID: 21774008]. https://doi.org/10.1002/ptr.3566.
-
20.
Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24(7):990-4. [PubMed ID: 20013822]. https://doi.org/10.1002/ptr.3044.
-
21.
Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2013;16(1):64-72. [PubMed ID: 23638294].
-
22.
Hosseinzadeh H, Behravan J, Ramezani M, Ajgan K. Anti-tumor and cytotoxic evaluation of Crocus sativus L. stigma and petal extracts using brine shrimp and potato disc assays. J Med Plants. 2005;3(15):59-65.
-
23.
Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50(8):761-86. [PubMed ID: 20830635]. https://doi.org/10.1080/10408390902773003.
-
24.
Yoshino F, Yoshida A, Umigai N, Kubo K, Lee MC. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J Clin Biochem Nutr. 2011;49(3):182-7. [PubMed ID: 22128217]. https://doi.org/10.3164/jcbn.11-01.
-
25.
Abe K, Sugiura M, Shoyama Y, Saito H. Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. Brain Res. 1998;787(1):132-8. [PubMed ID: 9518580].
-
26.
Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;3(19):40-50.
-
27.
Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22(1):16. [PubMed ID: 24401376]. https://doi.org/10.1186/2008-2231-22-16.
-
28.
Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H. Antidepressant Effect of Crocus sativus Aqueous Extract and its Effect on CREB, BDNF, and VGF Transcript and Protein Levels in Rat Hippocampus. Drug Res (Stuttg). 2015;65(7):337-43. [PubMed ID: 24696423]. https://doi.org/10.1055/s-0034-1371876.
-
29.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47-60. [PubMed ID: 6471907].
-
30.
D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60-90. [PubMed ID: 11516773].
-
31.
Lee B, Sur B, Shim I, Lee H, Hahm DH. Phellodendron amurense and Its Major Alkaloid Compound, Berberine Ameliorates Scopolamine-Induced Neuronal Impairment and Memory Dysfunction in Rats. Korean J Physiol Pharmacol. 2012;16(2):79-89. [PubMed ID: 22563252]. https://doi.org/10.4196/kjpp.2012.16.2.79.
-
32.
Saraf MK, Anand A, Prabhakar S. Scopolamine induced amnesia is reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2010;35(2):279-87. [PubMed ID: 19757037]. https://doi.org/10.1007/s11064-009-0051-4.
-
33.
Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667(1-3):222-9. [PubMed ID: 21616066]. https://doi.org/10.1016/j.ejphar.2011.05.012.
-
34.
Abe K, Sugiura M, Yamaguchi S, Shoyama Y, Saito H. Saffron extract prevents acetaldehyde-induced inhibition of long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999;851(1-2):287-9. [PubMed ID: 10642859].
-
35.
Yulug B, Ozan E, Gonul AS, Kilic E. Brain-derived neurotrophic factor, stress and depression: a minireview. Brain Res Bull. 2009;78(6):267-9. [PubMed ID: 19111910]. https://doi.org/10.1016/j.brainresbull.2008.12.002.
-
36.
Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23(34):10800-8. [PubMed ID: 14645472].
-
37.
Carlezon WJ, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436-45. [PubMed ID: 15982754]. https://doi.org/10.1016/j.tins.2005.06.005.
-
38.
Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22(9):3673-82. [PubMed ID: 11978843].
-
39.
Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33(5):230-40. [PubMed ID: 20223527]. https://doi.org/10.1016/j.tins.2010.02.001.
-
40.
Scott Bitner R. Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer's disease therapeutics. Biochem Pharmacol. 2012;83(6):705-14. [PubMed ID: 22119240]. https://doi.org/10.1016/j.bcp.2011.11.009.