Abstract
Background:
Arsenic trioxide (As2O3) is used for treating patients with acute promyelocytic leukemia (APL). The extensive application of this drug has been limited due to its severe cardiotoxicity. Montelukast is a selective leukotriene receptor antagonist with antioxidant and anti-inflammatory properties.Objectives:
This study evaluated whether montelukast could protect against As2O3-induced cardiotoxicity in vivo.Methods:
Thirty-two male Wistar rats (150 to 200 g) were divided to 4 treatment groups: control (0.2 mL of saline, ip), As2O3 (5 mg/kg, ip), As2O3 plus MONT, and MONT (20 mg/kg, po). All drugs were administered daily for 10 days and pretreatment with MONT was performed 1 hour prior to As2O3 administration. Cardiotoxicity was estimated by electrocardiological, biochemical, and histopathological evaluations.Results:
The results indicated that the combination treatment of montelukast and As2O3 markedly (P < 0.05) inhibited As2O3-induced lipid peroxidation and attenuated QT interval (QT) prolongation and histopathological alterations. Pretreatment with montelukast also suppressed increased troponin I and creatinine kinase-muscle brain (CK-MB) isoenzyme levels in response to As2O3 (P < 0.01).Conclusions:
In conclusion, the current results demonstrated that montelukast possesses beneficial cardio-protective properties against As2O3 toxicity. It was also proposed that these protective effects were mediated by antioxidant modifications of montelukast.Keywords
1. Background
Arsenic trioxide (As2O3) has been used in Chinese medicine for over 2500 years. It has been utilized successfully in the treatment of diseases, such as syphilis, tuberculosis, and malaria fever (1). Furthermore, As2O3 was approved by the United States food and drug administration (FDA) in 2000 for treating patients with hematological malignancies particularly for relapsed/refractory acute promyelocytic leukemia (APL) that is unresponsive to All-Trans retinoic acid (ATRA) (1-3). It may also be effective for some solid tumors in combination with other chemotherapeutic agents (1). Unfortunately, the use of this anti-neoplastic agent has been limited due to its fatal adverse effects, such as severe cardiotoxicity, hepatotoxicity, renal disorders, neurological defects, and type 2 diabetes (4). Cardiac abnormalities caused by As2O3, include QT interval (QT) prolongation, ventricular tachyarrhythmia, hypertension, torsades de pointes, endothelial dysfunction, and sudden death (5, 6).
Many studies have shown that generation of reactive oxygen species (ROS) and the resultant oxidative stress are the main mechanisms for arsenic pathogenesis (7-9). Reactive oxygen species cause alterations in various transcription factors, such as activator protein 1 (AP-1), Nuclear Factor Kappa B (NF-κB), and nuclear factor erythroid 2-related factor 2 (Nrf2), which are involved in antioxidant defense system (4, 8, 10). In addition, oxidative stress causes damage to cellular components (e.g. DNA, proteins, and lipids) and their functions (11-13).
Therefore, medications with considerable antioxidant properties would be valuable against oxidative damages induced by arsenic (14).
Leukotrienes, LTC4, LTD4, and LTE4, are a family of eicosanoid inflammatory mediators derived via the 5-lipoxygenase pathway from arachidonic acid (15). They play critical roles in bronchoconstriction, airflow obstruction, edema, and bronchial asthma (16, 17). Many studies have demonstrated that anti-leukotriene agents possess ROS scavenging properties (18). Montelukast (MONT) is a potent leukotriene LTD4/E4 receptor antagonist (cysteinyl leukotriene receptor 1 antagonist), which has been recognized to treat bronchial asthma, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) (19).
2. Objectives
The aim of this study was to determine whether montelukast could protect against As2O3-induced cardiotoxicity in vivo in rat.
3. Methods
3.1. Drugs
As2O3, hematoxylin, and eosin were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ketamine and xylazine were purchased from Alfasan Co (Woderen- Holland). Montelukast was purchased from Sobhan Pharmaceutical Co., Tehran, Iran.
3.2. Animals
A total number of 32 male albino Wistar rats, weighing 150 g to 200 g were obtained from the animal house of Ahvaz Jundishapur University of Medical Sciences. The animals were kept under normal laboratory conditions of humidity (50%), temperature (22 ± 2°C) and a 12-hour light/dark cycle, and allowed free access to commercial rodent chow and water. This protocol was approved by the Local committee of animal ethics of Ahvaz Jundishapur University of Medical Sciences (No. AJUMS. REC. 1395.104), Ahvaz, Iran.
3.3. Experimental Design
Animals were divided to 4 groups (n = 8); group I: control rats treated with normal saline 0.9% (0.2 mL, Intraperitoneally (IP)) daily for a period of 10 days, group II: rats treated with montelukast (20 mg/kg, PO) daily for a period of 10 days, group III: rats treated with arsenic trioxide (5 mg/kg, IP) daily for a period of 10 days, group IV: rats pretreated with montelukast (20 mg/kg, PO) 1 hour prior to As2O3 (5 mg/kg, IP), daily for a period of 10 days.
3.4. Electrocardiogram Recording
On the first day before any treatment and 48 hours after the last dose (12th day), all the animals were anesthetized using combination of ketamine HCL (100 mg/kg, IP) and xylazine (5 mg/kg, IP), and fifteen minutes after anesthesia, standard bipolar limb lead II electrocardiogram was recorded for 5 minutes in order to assess the QT interval. The QT intervals are influenced by heart rate (R-R cycle length), therefore, heart rate correction is required in the analysis of repolarization duration. Bazett’s formula is used for correcting the QT interval in the electrocardiogram for heart rate (R-R interval) (20).
Bazett’s formula:
QTc (QT corrected for HR) = QT interval/ √ RR interval
Lead II ECG was recorded by Bio-Amp and monitored by a Power Lab system (AD-Instruments, Australia).
3.5. Biochemical Measurements
After recording the secondary electrocardiogram (12th day), animals were sacrificed under mild anesthesia with combination of ketamine and xylazine and blood samples were drawn by heart puncture. Serums were separated from the blood samples and utilized for the biochemical examinations. After blood sampling, hearts were dissected and excised in ice cold conditions. Then, they were weighed and 0.1 g of heart tissues were homogenized using a homogenizer (Heidolph Silent Crusher M, Germany) in 1 mL PBS (50 mM, pH = 7.4) and centrifuged at 3000 rpm for 15 minutes. The clear supernatants were collected and utilized for the evaluation of glutathione peroxidase (GPx) activity and lipid peroxidation extent.
3.6. Measurement of Cardiac Markers
Troponin is a very specific and sensitive indicator of heart muscle damage. It is measured in the blood to diagnose various heart disorders, including unstable angina, myocardial infarction, and acute coronary syndrome (21, 22). The quantitative determination of cardiac troponin-I in the serum was performed using an immunodiagnostic kit, purchased from Monobind, Inc. Lake Forest, CA (92630), USA. Assays were performed following the kit instructions.
Creatinine kinase-muscle brain (CK-MB) isoenzyme in the serum was determined by a standard diagnostic kit from Pars Azmun Co, Iran.
3.7. GPx Activity
GPx acts as an endogenous antioxidant enzyme in numerous physiological processes. GPx catalyzes the reduction of hydrogen peroxide by reduced glutathione (GSH) and inhibits the oxidative stress insult. The activity of GPx was estimated using a standard diagnostic kit from ZellBio GmbH (Germany).
In brief, GPx uses reduced Glutathione (GSH) as an electron donor to convert it to glutathione oxidized (GSSG), and the remaining GSH can reduce 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB) and produce a yellow color. The GPx activity is indirectly related to color generation at 412 nm.
3.8. Lipid Peroxidation Assay
Malondialdehyde (MDA) levels were assayed as an indicator of lipid peroxidation in biological samples, using a standard diagnostic kit from ZellBio GmbH (Germany). In this protocol, MDA-Thiobarbituric acid (TBA) adduct was generated by the reaction of MDA and TBA under high temperature. Thiobarbituric acid-reactive substances were determined in acidic media and heat (90 - 100°C) colorimetrically at 535 nm.
3.9. Histopathology Examination
Heart tissue samples were fixed in formalin solution (10%) for 24 hours. The samples were then embedded in paraffin and tissue blocks were prepared. Heart tissue sections were stained by Hematoxylin and Eosin (H and E) and examined under a light microscope with 40× and 100× magnifications for morphological alterations.
3.10. Statistical Analysis
One way analysis of variance (ANOVA) was performed to analyze differences between groups followed by LSD and Tukey’s post hoc test for multiple comparisons. P values < 0.05 were considered statistically significant. All results were expressed as mean ± SD.
4. Results
4.1. Electrocardiogram Results
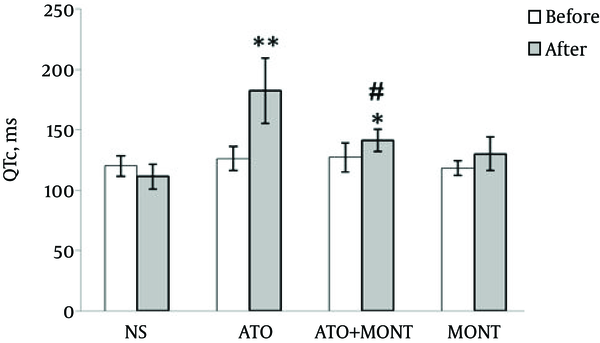
At the beginning of the study, before drug administrations, all rats presented normal electrocardiogram (ECG) patterns and similar QTc intervals in different groups. However, 48 hours following a period of 10 days of drug administrations, normal control and montelukast-treated groups showed a normal electrocardiogram pattern, whereas rats that had received As2O3 showed a marked increase in QTc interval as compared to the control group (P < 0.0001). As shown in Figure 1, pretreatment with montelukast (20 mg/kg, po) 1 hour prior to As2O3 injection resulted in a marked decrease in the QTc interval when compared to the As2O3-exposed group (P < 0.0001).
Values of QTc Intervals of Rats Based on Electrocardiogram Analysis
4.2. The Contents of Troponin I and CK-MB in Serum
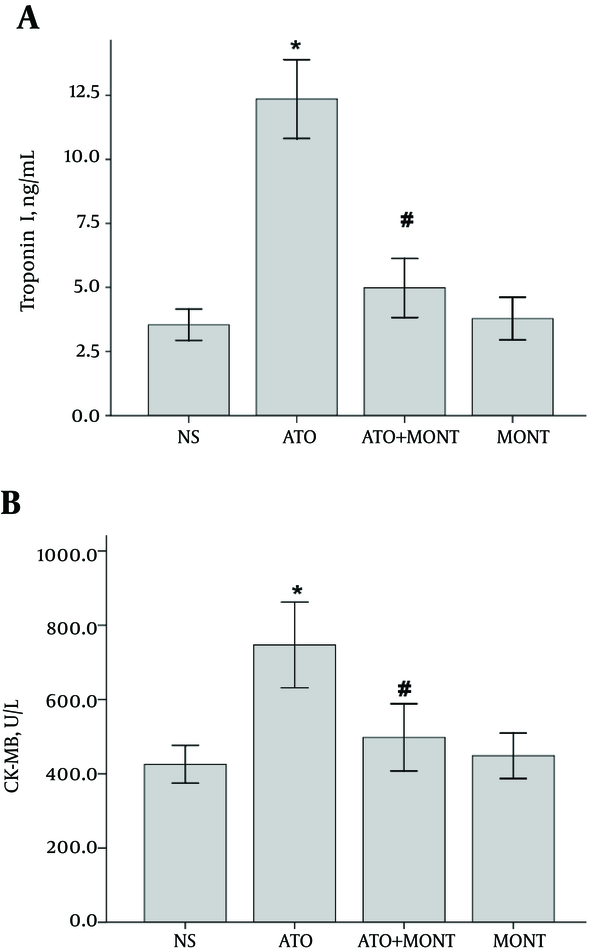
Administration of 5 mg/kg of As2O3 for 10 days caused a significant increase in the serum troponin-I and CK-MB levels compared to the control group (P < 0.0001). However, pretreatment with montelukast significantly, attenuated the elevations in serum troponin-I and CK-MB levels in comparison to the arsenic-treated group (P < 0.01). Administration of montelukast alone did not cause a significant change in the serum troponin-I and CK-MB contents (Figure 2).
Serum Levels of Cardiac Markers in Treated Groups
4.3. The Level of GPX Activity in Heart Tissue
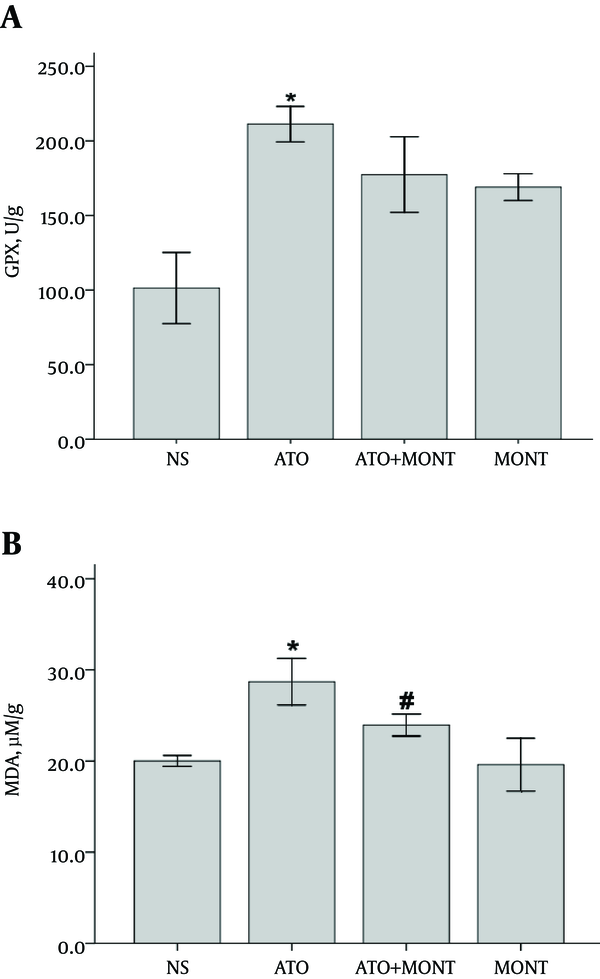
The GPX activity revealed a significant increase in heart homogenates of the As2O3-treated rats, when compared to the normal rats (P < 0.001). As shown in Figure 3A, pretreatment with montelukast (20 mg/kg, po) did not demonstrate a marked restoration in enzyme activity in the As2O3-exposed animals (P > 0.05).
Levels of Oxidative Stress Markers in Cardiac Tissue Homogenate
4.4. The Level of Lipid Peroxidation in Heart Tissue
The extent of lipid peroxidation increased in heart tissues of the As2O3-treated rats. It was revealed by a significant increase in MDA levels in this group when compared to normal rats (P < 0.001). Pretreatment with montelukast, effectively prevented this elevation in the As2O3-treated animals (P < 0.05) (Figure 3B).
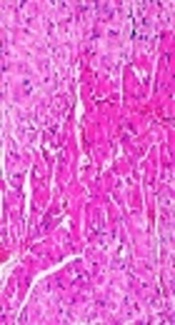
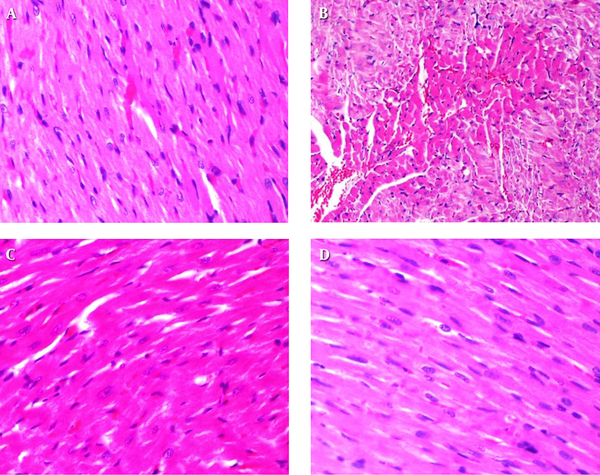
4.5. Effect of Montelukast on As2O3-Induced Cardiomyopathy
Results of histopathological examinations of myocardial tissues are shown in Figure 4. Heart tissue samples of control and montelukast-treated groups did not show morphological changes and normal myocardial tissues with clear nuclei were observed (Figure 4A, D). Moderate myocardial coagulative necrosis was detected in the heart samples of the As2O3-exposed animals as compared with those in the control group (Figure 4B). Co-treatment with montelukast, partly modulated morphological alterations in heart tissues of the As2O3-treated animals (Figure 4C).
Histopathologic View of Cardiac Tissue Sections Following Treatments
5. Discussion
Inorganic arsenic exposure increases ROS generation and cellular oxidative insults (4, 12). Imbalance between oxygen-derived radicals generation and cellular antioxidant capacity mediated by arsenic, plays a principal role in disease manifestation (1, 4).
In the cardiomyocytes, arsenic induces oxidative stress via ROS formation. The ROS decrease the expression of anti-apoptotic factors, such as Bcl-XL and Bcl-2, and increase the expression of pro-apoptotic factors, such as Bad, Bax, and Bid. Simultaneously, ROS also disrupt the mitochondrial membrane potential via calcium imbalance and open the membrane pores, which trigger complex events, such as release of cytosolic cytochrome c, activation of pro-caspase, and consequently caspase 3 leading to cellular apoptosis (4, 8). Therefore, chemicals with considerable antioxidant properties have a fundamental role in defense against As2O3-mediated free radicals and the resultant oxidative damages (14).
The current study investigated the beneficial effect of montelukst, leukotriene receptor blocker, on electrocardiogram pattern, stress oxidative parameters, serum cardiac markers, and histopathological alterations using the experimental model of As2O3-induced cardiotoxicity in rats.
The results indicated that QTc interval was prolonged in the electrocardiogram examination of As2O3-treated rats and oral pretreatment by montelukast attenuated this QTc prolongation (P < 0.0001). Pretreatment with montelukast also suppressed the elevated serum levels of cardiac markers, such as troponin I and CK-MB in response to As2O3 (P < 0.01).
Consistent with the current result,s an epidemiological study on Taiwanese cases living in an arsenic-exposed region demonstrated a direct correlation between QT prolongation in ECG pattern and tissue arsenic concentration (23). In another study, the prevalence of QTc prolongation in arsenic-exposed people was reported as 90% (24) and the underlying mechanisms of arsenic cardiotoxicity have been suggested as excessive generation of oxygen free radicals and oxidative stress (6, 24).
Numerous studies have shown that montelukast possesses considerable antioxidant and anti-inflammatory functions (16, 25). Protective effects of montelukast against oxidative stress have been attributed to a neutrophil-dependent pathway, ROS scavenging properties and inhibition of caspase 3 (14, 26, 27).
A recent study demonstrated that montelukast (20 mg/kg, IP) was effective in alleviating lipopolysaccharide-induced cardiac injury as evidenced by a decrease in serum levels of CK-MB, lactate dehydrogenase activity and alkaline phosphatase, and increase in GSH content in cardiac tissue homogenate with a concomitant decrease in MDA contents. Montelukast also decreased cardiac tumor necrosis factor α (TNF-α) expression and heart tissue morphological changes in comparison with the lipopolysaccharide group. This study suggested that the cardioprotective effects of montelukast could be attributed to its antioxidant and anti-inflammatory properties (28).
Based on the current results, montelukast may exert its cardioprotective effect against arsenic trioxide through its ROS scavenging properties and consequently, is capable of alleviating arsenic-induced changes in cardiac cells morphology, serum cardiac markers, and electrophysiological pattern.
The present found that As2O3 injection caused a marked increase in GPx activity and MDA formation in heart tissues (P < 0.001).
Recent studies have shown arsenic-induced Nrf2 activation. Nrf2 is a basic leucine zipper protein that attenuates arsenic-induced oxidative injury through increasing the gene expression of antioxidant enzymes, such as glutathione peroxidase, glutathione s-transferase, catalase and superoxide dismutase (4, 8). It seems that Nrf2 upregulates the gene expression of oxidative stress enzymes to rapidly return the induced enzymes to the baseline/normal levels or to maintain the endogenous antioxidant defense (8, 29).
A direct correlation between arsenic levels in tissue homogenate samples and lipid peroxidation was shown by several studies. Reactive oxygen species degrade poly unsaturated fatty acids and form MDA. The formation of MDA is used as an indirect biomarker to estimate the extent of oxidative stress in tissues (30, 31). In this study, pretreatment with montelukast could effectively decrease lipid peroxidation in the As2O3-exposed animals. The decrease in MDA levels may indicate montelukast as an effective ROS scavenger with potent antioxidant activity.
Histological evaluations revealed that As2O3 administration caused myocardial coagulative necrosis in all tissue sections. These observations are in accordance with previous studies that revealed histological alterations in heart tissue after arsenic injection (8, 32). Pretreatment with montelukast effectively prevented cardiomyocyte necrosis and preserved tissue morphology in As2O3-induced heart injury in rats, demonstrating the advantageous role of montelukast against As2O3- induced cardiotoxicity.
5.1. Conclusions
In summary, the findings of the current study propose that montelukast was effective in alleviating As2O3-induced myocardial injury as evidenced by preventing lipid peroxidation in heart tissue and decrease in serum troponin I and CK-MB levels. Additionally, montelukast prevented morphological changes in cardiac cells and consequently electrophysiological abnormalities. The valuable effects of montelukast, which have been observed in this work, provide a safe and inexpensive option for the prevention of cardiotoxicity of arsenic trioxide in patients with APL. However, such application of montelukast may need further clinical trials.
Acknowledgements
References
-
1.
Miao X, Tang Z, Wang Y, Su G, Sun W, Wei W, et al. Metallothionein prevention of arsenic trioxide-induced cardiac cell death is associated with its inhibition of mitogen-activated protein kinases activation in vitro and in vivo. Toxicol Lett. 2013;220(3):277-85. [PubMed ID: 23664956]. https://doi.org/10.1016/j.toxlet.2013.04.025.
-
2.
Zhang J, Wang B. Arsenic trioxide (As(2)O(3)) inhibits peritoneal invasion of ovarian carcinoma cells in vitro and in vivo. Gynecol Oncol. 2006;103(1):199-206. [PubMed ID: 16624393]. https://doi.org/10.1016/j.ygyno.2006.02.037.
-
3.
Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6 Suppl 2:1-2. [PubMed ID: 11331433]. https://doi.org/10.1634/theoncologist.6-suppl_2-1.
-
4.
Flora SJ. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51(2):257-81. [PubMed ID: 21554949]. https://doi.org/10.1016/j.freeradbiomed.2011.04.008.
-
5.
Zhao X, Feng T, Chen H, Shan H, Zhang Y, Lu Y, et al. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic Clin Pharmacol Toxicol. 2008;102(5):419-25. [PubMed ID: 18346055]. https://doi.org/10.1111/j.1742-7843.2007.00150.x.
-
6.
Shan H, Zhang Y, Cai B, Chen X, Fan Y, Yang L, et al. Upregulation of microRNA-1 and microRNA-133 contributes to arsenic-induced cardiac electrical remodeling. Int J Cardiol. 2013;167(6):2798-805. [PubMed ID: 22889704]. https://doi.org/10.1016/j.ijcard.2012.07.009.
-
7.
Kucukkurt I, Ince S, Demirel HH, Turkmen R, Akbel E, Celik Y. The Effects of Boron on Arsenic-Induced Lipid Peroxidation and Antioxidant Status in Male and Female Rats. J Biochem Mol Toxicol. 2015;29(12):564-71. [PubMed ID: 26184899]. https://doi.org/10.1002/jbt.21729.
-
8.
Saad SY, Alkharfy KM, Arafah MM. Cardiotoxic effects of arsenic trioxide/imatinib mesilate combination in rats. J Pharm Pharmacol. 2006;58(4):567-73. [PubMed ID: 16597375]. https://doi.org/10.1211/jpp.58.4.0017.
-
9.
Khodayar MJ, Javadipour M, Keshtzar E, Rezaei M. Role of berberine against arsenic induced oxidative damage in isolated rat liver mitochondria. J Environ Biol. 2016;37(2):285-90. [PubMed ID: 27097449].
-
10.
Dianat M, Amini N, Badavi M, Farbood Y. Ellagic acid improved arrhythmias induced by CaCL2 in the rat stress model. Avicenna J Phytomed. 2015;5(2):120-7. [PubMed ID: 25949953].
-
11.
Shafik NM, El Batsh MM. Protective Effects of Combined Selenium and Punica granatum Treatment on Some Inflammatory and Oxidative Stress Markers in Arsenic-Induced Hepatotoxicity in Rats. Biol Trace Elem Res. 2016;169(1):121-8. [PubMed ID: 26085057]. https://doi.org/10.1007/s12011-015-0397-1.
-
12.
De Vizcaya-Ruiz A, Barbier O, Ruiz-Ramos R, Cebrian ME. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat Res. 2009;674(1-2):85-92. [PubMed ID: 18984063]. https://doi.org/10.1016/j.mrgentox.2008.09.020.
-
13.
Badavi M, Sadeghi N, Dianat M, Samarbafzadeh A. Effects of gallic Acid and cyclosporine a on antioxidant capacity and cardiac markers of rat isolated heart after ischemia/reperfusion. Iran Red Crescent Med J. 2014;16(6). e16424. [PubMed ID: 25068044]. https://doi.org/10.5812/ircmj.16424.
-
14.
Ahmed AA. Protective effect of montelukast on paraquat-induced lung toxicity in rats. Biosci Trends. 2009;3(2):63-72. [PubMed ID: 20103949].
-
15.
Hemmati AA, Ghorbanzadeh B, Behmanesh MA. Potentiation of indomethacin-induced anti-inflammatory response by montelukast in formalin-induced inflammation in rats. Acta Med Iran. 2013;51(10):675-80. [PubMed ID: 24338138].
-
16.
Cuciureanu M, Caruntu ID, Paduraru O, Stoica B, Jerca L, Crauciuc E, et al. The protective effect of montelukast sodium on carbon tetrachloride induced hepatopathy in rat. Prostaglandins Other Lipid Mediat. 2009;88(3-4):82-8. [PubMed ID: 19041730]. https://doi.org/10.1016/j.prostaglandins.2008.10.004.
-
17.
Kuru S, Kismet K, Barlas AM, Tuncal S, Celepli P, Surer H, et al. The Effect of Montelukast on Liver Damage in an Experimental Obstructive Jaundice Model. Viszeralmedizin. 2015;31(2):131-8. [PubMed ID: 26989383]. https://doi.org/10.1159/000375434.
-
18.
Al Saadi MM, Meo SA, Mustafa A, Shafi A, Tuwajri AS. Effects of Montelukast on free radical production in whole blood and isolated human polymorphonuclear neutrophils (PMNs) in asthmatic children. Saudi Pharm J. 2011;19(4):215-20. [PubMed ID: 23960762]. https://doi.org/10.1016/j.jsps.2011.06.002.
-
19.
Joos S, Miksch A, Szecsenyi J, Wieseler B, Grouven U, Kaiser T, et al. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: a systematic review. Thorax. 2008;63(5):453-62. [PubMed ID: 18443162]. https://doi.org/10.1136/thx.2007.081596.
-
20.
Shah S, Mohan M, Kasture S, Sanna C, Maxia A. Protective effect of Ephedra nebrodensis on doxorubicin-induced cardiotoxicity in rats. Iran J Pharmacol Ther. 2009;8(2):61-6.
-
21.
Robertson IM, Sun YB, Li MX, Sykes BD. A structural and functional perspective into the mechanism of Ca2+-sensitizers that target the cardiac troponin complex. J Mol Cell Cardiol. 2010;49(6):1031-41. [PubMed ID: 20801130]. https://doi.org/10.1016/j.yjmcc.2010.08.019.
-
22.
Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424(6944):35-41. [PubMed ID: 12840750]. https://doi.org/10.1038/nature01780.
-
23.
Wang CH, Chen CL, Hsiao CK, Chiang FT, Hsu LI, Chiou HY, et al. Arsenic-induced QT dispersion is associated with atherosclerotic diseases and predicts long-term cardiovascular mortality in subjects with previous exposure to arsenic: A 17-Year follow-up study. Cardiovasc Toxicol. 2010;10(1):17-26. [PubMed ID: 19957052]. https://doi.org/10.1007/s12012-009-9059-x.
-
24.
Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation. 2004;109(1):26-9. [PubMed ID: 14691044]. https://doi.org/10.1161/01.CIR.0000109484.00668.CE.
-
25.
Dengiz GO, Odabasoglu F, Halici Z, Cadirci E, Suleyman H. Gastroprotective and antioxidant effects of montelukast on indomethacin-induced gastric ulcer in rats. J Pharmacol Sci. 2007;105(1):94-102. [PubMed ID: 17895592]. https://doi.org/10.1254/jphs.FP0070122.
-
26.
Ersahin M, Cevik O, Akakin D, Sener A, Ozbay L, Yegen BC, et al. Montelukast inhibits caspase-3 activity and ameliorates oxidative damage in the spinal cord and urinary bladder of rats with spinal cord injury. Prostaglandins Other Lipid Mediat. 2012;99(3-4):131-9. [PubMed ID: 22986158]. https://doi.org/10.1016/j.prostaglandins.2012.09.002.
-
27.
Anderson R, Theron AJ, Gravett CM, Steel HC, Tintinger GR, Feldman C. Montelukast inhibits neutrophil pro-inflammatory activity by a cyclic AMP-dependent mechanism. Br J Pharmacol. 2009;156(1):105-15. [PubMed ID: 19068077]. https://doi.org/10.1111/j.1476-5381.2008.00012.x.
-
28.
Khodir AE, Ghoneim HA, Rahim MA, Suddek GM. Montelukast attenuates lipopolysaccharide-induced cardiac injury in rats. Hum Exp Toxicol. 2016;35(4):388-97. [PubMed ID: 26089034]. https://doi.org/10.1177/0960327115591372.
-
29.
Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36(10):1199-207. [PubMed ID: 15110384]. https://doi.org/10.1016/j.freeradbiomed.2004.02.074.
-
30.
Cooper KL, Liu KJ, Hudson LG. Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase. Free Radic Biol Med. 2009;47(4):381-8. [PubMed ID: 19414066]. https://doi.org/10.1016/j.freeradbiomed.2009.04.034.
-
31.
Flora SJ, Mehta A, Gupta R. Prevention of arsenic-induced hepatic apoptosis by concomitant administration of garlic extracts in mice. Chem Biol Interact. 2009;177(3):227-33. [PubMed ID: 18834867]. https://doi.org/10.1016/j.cbi.2008.08.017.
-
32.
Manna P, Sinha M, Sil PC. Arsenic-induced oxidative myocardial injury: protective role of arjunolic acid. Arch Toxicol. 2008;82(3):137-49. [PubMed ID: 18197399]. https://doi.org/10.1007/s00204-007-0272-8.