Abstract
Background:
Herbal antioxidants have beneficial effects on health and performance. Meanwhile, medicinal plants and their derivations are utilized as growth and immunity promoter.Objectives:
This research aimed to evaluate the effects of garlic powder and Satureja Khuzestanica essential oil (SKEO) on broiler performance, blood lipid profile, immune responses, intestinal microflora, and morphology.Methods:
In total, 400 male chickens were randomly divided into five groups. Treatments included garlic powder (2 and 4%) and SKEO (400 and 500 mg/kg). Those in the control group received no intervention. On the 35th day of the experiment, the immune system of subjects was investigated, and at the end of the study, performance and blood lipid profile were analyzed. Also, the jejunal and ileal contents were separated to investigate the intestinal morphology and microflora.Results:
Administration of herbal extracts was associated with improved performance, such as feed intake, BWG, and FCR (P < 0.05). Treatments could diminish the serum concentrations of lipid profile, including cholesterol, triglycerides, and LDL (P < 0.05). Meanwhile, the number of harmful intestinal bacteria was reduced by garlic (4% of diet) and SKEO (500 mg/kg) treatments (P < 0.05). Also, morphological characteristics of the intestine were improved (P < 0.05). Herbal plant supplement remarkably increased the villus length, villus length to crypt depth, and villus area (P < 0.05), but did not affect the immune responses (P > 0.05).Conclusions:
This study demonstrated that dietary inclusion of herbal extracts is potentially an effective strategy for improving health and performance in broiler chickens.Keywords
Broiler Performance Garlic Lipid Profile Microflora Morphology Satureja
1. Background
Increased human population has caused enhanced demand for animal products. Hence, poultry breeders are looking for alternative dietary energy and protein to be used in human food and animal feed (1, 2). Meanwhile, regarding the recent concerns about the limitations of using synthetic antibiotics for increasing the growth and immunity modulation in chicken breeding, there is a growing interest in reducing antibiotic resistance using the herbal additive. Herbal plant supplements are of main economic significance for poultry breeders. Thus, the use of herbals and their derivatives may be an appropriate strategy for improving the health and performance of poultry.
Garlic (Allium sativum L.) is a medicinal plant belonging to the versatile herbs with numerous applications in the food and drug industries. Basically, plant derivatives (from garlic) are usually utilized in bird feeding as a growth promoter, because of their antioxidant, antimicrobial, and digestion properties (3-5). Further, Puvača et al. (6) showed that dietary inclusion of spice herbs (garlic, black pepper, and hot red pepper) leads to higher production levels and much better blood lipid profile conditions.
Satureja Khuzestanica is a medicinal herb belonging to the Lamiaceae family, which is mainly cultivated for culinary uses and medical all around the world. Satureja Khuzestanica Jamzad (SKEO), also known as Marzeh-e-Khuzestani in Persian, is an endemic plant and native to southern parts of Iran. The main constituents of the SKEO are carvacrol (more than 90%), followed by flavones, triterpenoids, tannins, and steroids (7). Recent studies reported that SKEO may have economic benefits in broiler flocks under heat stress through improved feed conversion ratio (FCR) and efficiency factor (8) by promoting the digestion process (9). In addition, some studies revealed that SKEO into diet resulted in beneficial actions on antioxidant properties (10-12). Further, Nasimi et al. (7) showed that SKEO could prevent oxidative changes created via reactive oxygen species (ROS) by lipid peroxidation inhibition and radical scavenging, as well as their total phenolic compounds.
2. Objectives
The current study aimed to test whether garlic powder and SKEO can protect the health and performance of broiler chickens via modifying lipid profile and microflora state.
3. Methods
3.1. Preparation of Extracts
The garlic powder and SKEO used in this study were purchased from the Known Technology Pharmaceuticals Co. (Tehran, Iran). The volume of extracts was measured by the Folin-Ciocalteu reagent (13). SKEO contained 24.5% p-Cymene and 39.74% carvacrol phenolic compounds.
3.2. Birds and Experimental Procedure
In the present study, 400 one-day-old male chickens (42 ± 3.0 g) were obtained from a commercial hatchery (Toyoorbarekat Co. Tehran, Iran). Then, chickens were randomly divided into 5 groups. Treatments included garlic powder (2 and 4%) and SKEO (400 and 500 mg/kg). Those in the control group did not receive any intervention. Diets and water were available ad libitum during the experiment. For meeting their nutritional needs, three dietary formulations were used during growth periods, as recommended by National Research Council (NRC) NRC (14) (Table 1). The control group did not receive any feed additives, whereas in diets containing levels of SKEO, this additive was administered to birds at two levels of 400 and 500 mg/kg mixed with the basal diet (Table 1), and the other groups received 2% and 4% of garlic powder (Table 2). A sample of the diets was analyzed according to the method proposed by Hadian et al. (9). The composition of SKEO is provided in Table 3. The study protocol was performed in compliance with the guidelines for handling farm animals, which was approved by the Ethics Committee of the Islamic Azad University, Arak Branch, Iran (code: 98-02-32-51985).
| Ingredients (%) | Periods | ||
|---|---|---|---|
| Starter (0 - 10 Days) | Grower (11 - 24 Days) | Finisher (25 - 42 Days) | |
| Corn (8.5% CP) | 54.32 | 58 | 66 |
| Soybean meal (44% CP) | 39.80 | 35.22 | 29.00 |
| Soybean oil | 2.15 | 3.30 | 2.00 |
| Oyster shell | 0.8 | 0.70 | 0.65 |
| Di-calcium phosphate | 1.75 | 1.65 | 1.30 |
| Vitamin and Mineral premix | 0.50 | 0.50 | 0.50 |
| Salt | 0.23 | 0.23 | 0.20 |
| DL-methionine and L-Lysine | 0.45 | 0.40 | 0.35 |
| Chemical component | |||
| Metabonlizable energy (kcal/kg) | 2980 | 3100 | 3150 |
| Protein (%) | 23.00 | 21.24 | 19.08 |
| Methionine + Lysine (%) | 1.80 | 1.73 | 1.51 |
| Methionine + cysteine (%) | 0.95 | 0.81 | 0.76 |
| Calcium + Phosphorus (%) | 1.44 | 1.34 | 1.13 |
Composition of Experimental Diets Containing Garlic Powder in The Different Periods (As-Fed Basis)
| Ingredients (%) | Periods | |||||
|---|---|---|---|---|---|---|
| Starter (0 - 10 Days) | Grower (11 - 24 Days) | Finisher (25 - 42 Days) | ||||
| Garlic Powder Levels | ||||||
| 2% | 4% | 2% | 4% | 2% | 4% | |
| Corn (8.5% CP) | 54 | 53 | 58 | 87 | 59.7 | 58 |
| Soybean meal (44% CP) | 38.3 | 38.3 | 33.6 | 32 | 31 | 31 |
| Galic Powder | 2 | 4 | 2 | 4 | 2 | 4 |
| Soya oil | 2 | 2 | 3 | 3 | 4 | 4 |
| Oyster shell | 0.8 | 0.8 | 0.61 | 0.61 | 0.61 | 0.61 |
| Di-calcium phosphate | 1.75 | 1.75 | 1.65 | 1.65 | 1.65 | 1.4 |
| Vitamin and Mineral premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| DL-methionine and L-Lysine | 0.45 | 0.45 | 0.33 | 0.33 | 0.33 | 0.26 |
| Chemical analysis | ||||||
| Metabolizable energy (kcal/kg) | 2920 | 2870 | 3030 | 2980 | 3110 | 3050 |
| Crude protein (%) | 22.41 | 22 | 20.6 | 20 | 19.5 | 19.5 |
| Methionine + Lysine (%) | 1.88 | 1.84 | 1.63 | 1.58 | 1.57 | 1.52 |
| Methionine + cysteine (%) | 0.91 | 0.9 | 0.77 | 0.75 | 0.75 | 0.71 |
| Calcium + Phosphorus (%) | 1.44 | 1.44 | 1.31 | 1.31 | 1.09 | 1.17 |
Composition of Satureja Khuzestanica Essential Oil
| Chemical Compound | Composition (%) |
|---|---|
| Carvacrol | 92.16 |
| (Z)-β-ocimene | 0.54 |
| Myrcene | 0.26 |
| α-pinene | 0.15 |
| α-terpinene | 0.24 |
| α-thujene | 0.24 |
| P-cymene | 1.26 |
| Limonene | 0.13 |
| γ-terienene | 0.74 |
| trans-sabinene hydrate | 0.17 |
| α-terpinolene | 0.42 |
| β-caryophyllene | 0.16 |
| Trans-β-bisabolene | 0.10 |
3.3. Growth Performance
The chickens were weighed at the beginning of the study and at the end, and the body weight gain (BWG) per replicate was analyzed. Feed intake (FI) index was recorded for each replicate, and the FCR was calculated.
3.4. Blood Analysis
Two chickens were randomly selected from each pen for blood sample analyses at 42 days of age. The serum was separated by centrifugation (15 min at 4°C and 2500×g), kept at -20°C for later analyses. Concentrations of serum lipids, including cholesterol, triglycerides, and other lipid factors in the blood such as low-density lipoprotein (LDL) and high-density lipoprotein (HDL), were evaluated using atomic absorption spectrophotometer (Perkin-Elmer, AA-600, USA). Standard commercial kits were used for analysis (Pars Azmoon Co. Tehran, Iran).
3.5. Immune Responses
After 28 days of the experiment, 2 birds were selected for injecting sheep red blood cells (SRBC). The anti-SRBC antibody titers were measured using the method proposed by Qureshi and Havanstein (15). Antibody data are presented as log2 of the reciprocal of the highest dilution yielding visible agglutination.
3.6. Intestinal Morphometry
At the end of the study, 2 chickens were killed for intestinal studies. For this aim, 1.5 cm of the jejunum was sampled and washed with 85% saline for 24 h and were placed in formalin (10%). Routine histological laboratory methods were used. Six micrometer thick sections were stained with Hematoxilin-Eosin and were observed under a microscope. After preparing the samples, intestinal performances such as villus length and width, villus length-crypt depth ratio (VH/CD), and crypt depth were measured using Iji et al. (16) methods, by a light microscope (Olympus Co. Tokyo, Japan). Calculations were determined by the following formula: [2π × (W/2) × L], where W = villus width, L = villus length.
3.7. Ileal Bacterial Study
At the end of the experiment, 1 gram of ileal content obtained from two chicks was collected and transferred to 9 mL (NaCl, 9 g L−1). All procedures for ileal bacterial were performed according to Mookiah et al. (17). Agar (Oxoid) for total aerobic bacteria, plates inoculated for enumeration incubated anaerobically at 37°C for 48 h in anaerobic jars (Oxoid) with anaeroGen GasPak (Oxoid), and plates for E. coli and total aerobes were incubated aerobically at 37°C for 24 h, after which total numbers of bacterial colonies were counted. Bacterial populations were expressed as log10 colony forming units (cfu) g−1 caecal content. All procedures were performed in duplicate (17).
3.8. Statistical Analysis
The effects of medical plants were evaluated by a completely randomized design. Data were analyzed by ANOVA using the GLM procedure of SAS (2005). Treatments were compared by Duncan’s test, and the differences were separated at the statistical level of P < 0.05 (18).
4. Results
4.1. Growth Performance
The results related to chickens' performance are presented in Table 4. Data indicated that herbal plant supplements could significantly promote BWG, FI, and FCR (P < 0.05), but no difference was observed concerning the mortality rate (P > 0.05).
Effects of Dietary Inclusion of Garlic Powder and Satureja Khuzestanica Essential Oil (SKEO) on Feed Intake (FI; G/Chick), BWG (G/Chick), FCR (G Feed/G BWG), And Mortality (%) Of Broiler Chickens a, b
| Groups | FI | BWG | FCR | Mortality |
|---|---|---|---|---|
| Control | 3901.00 ± 141.40c | 1753.00 ± 58.45d | 2.23 ± 0.09a | 5.32 ± 4.91 |
| Garlic 2% | 3944.00 ± 128.10c | 1771.00 ± 82.16d | 2.19 ± 0.09a | 6.67 ± 5.16 |
| Garlic 4% | 4297.00 ± 66.46b | 2368.00 ± 40.82b | 1.76 ± 0.03c | 5.00 ± 5.47 |
| SKEO (400 mg/kg) | 4019.00 ± 75.28c | 2102.00 ± 111.40c | 1.95 ± 0.08b | 4.98 ± 4.66 |
| SKEO (500 mg/kg) | 4718.00 ± 75.28a | 2602.00 ± 91.74a | 1.75 ± 0.04c | 6.21 ± 5.35 |
| P Value | 0.001 | 0.001 | 0.001 | 0.66 |
| SEM | 56.23 | 64.05 | 0.037 | 0.78 |
4.2. Blood Analysis
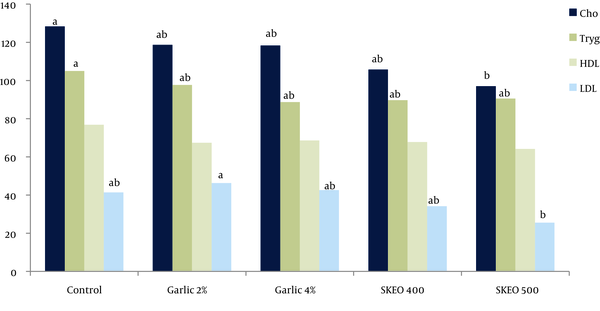
The blood lipid profile of each group is provided in Figure 1. Chickens treated with garlic (4% of diet) and SKEO (500 mg/kg) showed lower serum concentrations of cholesterol, triglycerides, and LDL compared to other birds (P < 0.05). No significant difference was observed between the two groups concerning serum concentrations of HDL (P > 0.05).
Effects of dietary inclusion of garlic powder and Satureja Khuzestanica essential oil (SKEO) on blood lipid profile (mg/dL) of broiler
4.3. Immune Responses
The effects of dietary supplementation with garlic powder and SKEO on humoral immunity are provided in Table 5. According to the findings of the present study, experimental diets did not affect the immune responses of broiler chickens (P > 0.05).
Effects of Dietary Inclusion of Garlic Powder and Satureja Khuzestanica Essential Oil (SKEO) on SRBC Antibody Titer (Log10) of Broiler Chickens
| Trait | Treatments | Statistical Parameters | |||||
|---|---|---|---|---|---|---|---|
| Control | Garlic 2% | Garlic 4% | SKEO (400 mg/kg) | SKEO (500 mg/kg) | P Value | SEM | |
| SRBC antibody titer | 5.51 | 5.03 | 4.52 | 6.21 | 7.23 | 0.37 | 0.76 |
4.4. Ileal Bacterial Populations
Table 6 shows the effects of diets on the intestinal microbial and morphology. A significantly lower illeal content of Coliform bacteria, E. coli, and total aerobic bacteria was found in the treatment groups (P < 0.05) compared to the controls. The experimental diets presented higher villus length, VH/CD, and villus area compared to unchallenged birds (Figure 2). At the same time, our findings showed that diets affected the depth and villus width (P > 0.05).
Effects of Dietary Inclusion of Garlic Powder and Satureja Khuzestanica Essential Oil (SKEO) on Intestinal Microflora and Morphology of Broiler Chickens a, b
| Traits | Treatments | Statistical Parameters | |||||
|---|---|---|---|---|---|---|---|
| Control | Garlic 2% | Garlic 4% | SKEO (400 mg/kg) | SKEO (500 mg/kg) | SEM | P-Value | |
| Intestinal microflora log10 (CFU/g) | |||||||
| E. coli | 3.93A | 3.43A | 2.73B | 3.63A | 1.87C | 0.14 | 0.03 |
| Total of aerobic bacteria | 11.29A | 9.43A | 7.95B | 8.47AB | 6.21C | 0.37 | 0.04 |
| Coliform bacteria | 7.42A | 2.22C | 4.68B | 4.79B | 2.16C | 0.54 | 0.01 |
| Morphology | |||||||
| Villus length (µm) | 1142.00C | 1287.6B | 1321.4AB | 1231.6AB | 1303.2A | 28.85 | 0.001 |
| Villus width (µm) | 118.32 | 110.31 | 112.81 | 105.29 | 117.48 | 4.93 | 0.349 |
| Crypt depth (µm) | 125.00 | 121.23 | 117.31 | 113.62 | 128.67 | 6.60 | 0.512 |
| VH/CD | 9.39B | 11.18A | 11.95A | 10.00A | 11.65A | 0.376 | 0.011 |
| Villus area (mm2) | 0.435B | 0.429AB | 0.485AB | 0.483AB | 0.509A | 0.025 | 0.024 |
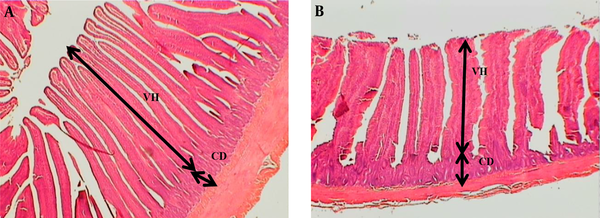
The part of a jeojenom tissue of broilers A: Broiler received 500mg/kg SKEO, B: Control treatment with no feed additives. In the control group, the villus structures are short and thickened compared to the birds fed with 500 mg/kg SKEO. CD = Crypt depth, VH = Villi height. Hematoxylin and eosin staining.
5. Discussion
Recently more attention is paid to improving the performance of poultry using medical plant extract supplementations. Meanwhile, the use of medicinal herbs, as a growth promoter, has considerably increased, due to the several benefits including their natural constituents, and low price, safety, and availability (3-5). In the present study, the difference in performance, blood lipid profile, immune responses, intestinal microflora, and morphology between the control and treated groups can be attributed to differences in daily content of the diet and stable general condition of the birds. It means that changing the diet could affect the health and performance subjects, which consequently changed the quality of chicken's products.
The present study proved that dietary inclusion of medical plant supplements could improve BWG and FCR of the broiler chickens (Table 4). Our observations showed the development of poultry breeders, both in productive and herbal extract, because natural products do not generate harmful residues in food and often do not cause problems such as antibacterial resistance (19). Previous studies reported that supplementation of garlic powder improved the performance of broilers when added at rates of 1% (20) and 3% (21, 22) to broiler's diets. Thus, it can be an alternative for antibiotic growth promoters. In the same vein, Lee et al. (23) reported that an increase in BWG of chickens after using 0.2 g/kg carvacrol. In contrast to these results, the latest studies have shown that dietary inclusion of garlic essential oils did not have any beneficial effect on BWG, FI, and FCR (24, 25). Khosravinia et al. (9) indicated that the performance parameters were not affected after SKEO supplementation in drinking water, which is not in line with the findings of the present study. This difference can be attributed to the associated and variability of new feed additives levels as well as environmental conditions (23) and physiological status (9). Additionally, the inconsistency in the results of the present study with other research can be attributed to the fact that in the current study, SKEO supplementation was added to the diet, while Khosravinia et al. (9) have used drinking water. It seems that herbal extract supplementations increased the appetite of subjects. Besides, there was a direct association between the trend of FI and the level of garlic powder and SKEO in the diet. In addition, some studies showed that improved growth performance indexes in high levels of garlic powder and SKEO can be due to antioxidant properties. Also, phenolic components of garlic and SKEO supplements not only can reduce the effects of pathogens on the intestinal system but also are useful for absorbing amino acids in the small intestinal (5, 11, 12).
Because the chickens treated with garlic (4% of diet) and SKEO (500 mg/kg) had lower serum concentrations of cholesterol, triglycerides, and LDL, accordingly using herbal plant extracts could improve the chickens' health and productivity by regulating lipid profiles. The latest studies showed that dietary inclusion of garlic powder (6, 26) or SKEO (9) stimulates a better lipid profile in broiler chickens, which might be attributed to their furanocoumarins and flavonoids properties. Moreover, adding small amounts of tellurium to garlic powder can decrease endogenous cholesterol production via inhibition of hepatic squalene epoxidase (27). Also, carvacrol, as a monophenolic molecule, can affect LDL and/or HDL metabolism via extrahepatic metabolic paths (28). Garlic, as an effective antioxidant, could reduce cholesterol content by affecting hepatic 3-hydroxy-3-methyl glutaryl coenzyme A reductase, which in turn reduces cholesterol biosynthesis enzymes (27). On the other hand, based on the results related to blood lipid profile, decreased cholesterol synthesis following supplementation with garlic powder or SKEO in diet, may be responsible for decreased LDL synthesis, because cholesterol is one of the precursors for LDL synthesis. Cholesterol in birds is often in the form of HDL (α-2globulin fraction) and LDL (β-2globulin fraction) (29).
Some researchers reported that garlic powder (30) and SKEO (31) exhibited significant hypocholesterolemic and hypolipidemic actions in broiler chickens, which is consistent with our results. Medical plant extracts can alter the immune function by dynamic regulation of molecules like cytokines and chemokines (30). In the current study, herbal extracts did not affect the SRBC antibody titer. Furthermore, some studies reported that phytogenics supplementation did not affect total antibody titer, which is consistent with the findings of the present study (32). Amresh et al. (33) showed that flavonoids and polyphenolic compounds in herbal plants could strengthen the immune system because of their antioxidant properties. Additionally, previous studies have shown that dietary inclusion of garlic powder increased cellular and humoral immune systems of broiler chickens (22, 34), which might be imputated to its amount in furanocoumarins and flavonoids. The immune response of chickens to phytogenic essential oils might be affected by the animal's age and gender, hygiene, environmental factors, the composition of the feed, and quality of products (35, 36).
Controlling the growth and numbers of harmful bacteria is a vital objective for the poultry industry. In the present study, adding herbal plant extracts to experimental diets could improve poultry health and product performance by compilation of intestinal microbial flora. The latest studies reported that some herbal antioxidants can increase the number of beneficial bacteria and suppress pathogenic bacteria colonization (25, 37). Sharifi-Rad et al. (38) reported slight antibacterial activities of SKEO against intestinal microbes. The antibacterial properties of the essential oils are because fat can pierce the bacterial membrane and expels the cell components (37). It worth noting that the antibacterial properties of essential oils in poultry may be changed by interfering with diet and altering environmental conditions (25). In short, adding medical plant extracts to the poultry diet is an economical way to increase the intestinal microbial population.
Our morphology findings indicated that herbal extracts exposure was associated with increased villus length, VH/CD, and villus in the male chickens, which in turn causes better absorption of nutrients in the gut. These findings are consistent with the results of other studies, which reported that adding herbal plant extracts could increase the villus length (26, 32) and VH/CD ratio (37, 38). In addition, Amad et al. (39) showed that supplementation of herbal plant additive to poultry diet could change jejunal tissue, which is useful for better absorbing nutrients and improving functions such as FCR. Meanwhile, according to Viveros et al. (40), phenolic derivatives can alter the intestinal microbial ecology in poultry and cause positive changes in broiler intestinal bacteria.
5.1. Conclusions
In summary, this study demonstrated that using garlic powder and SKEO compounds is potentially an effective strategy for modifying the blood lipid profile and suppressing bacterial populations at the broiler chickens via antioxidant functions. However, a full understanding of the mechanism by which these herbal plant extracts function is still lacking.
References
-
1.
FAO/OECD. OECD-FAO Agricultural Outlook 2015-2024. 2016.
-
2.
Altmann BA, Neumann C, Velten S, Liebert F, Morlein D. Meat quality derived from high inclusion of a micro-alga or insect meal as an alternative protein source in poultry diets: A pilot study. Foods. 2018;7(3). [PubMed ID: 29518048]. [PubMed Central ID: PMC5867549]. https://doi.org/10.3390/foods7030034.
-
3.
Ordóñez G, Llopis N, Peñalver P. Efficacy of eugenol against a salmonella enterica serovar enteritidis experimental infection in commercial layers in production. J Appl Poultry Res. 2008;17(3):376-82. https://doi.org/10.3382/japr.2007-00109.
-
4.
Hanieh H, Narabara K, Piao M, Gerile C, Abe A, Kondo Y. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim Sci J. 2010;81(6):673-80. [PubMed ID: 21108687]. https://doi.org/10.1111/j.1740-0929.2010.00798.x.
-
5.
Al-Shuwaili MA, Ibrahim EI, Naqi Al-Bayati MT. Effect of dietary herbal plants supplement in turkey diet on performance and some blood biochemical parameters. Glob J Biosci Biotechnol. 2015;4(2):153-7.
-
6.
Puvača N, Kostadinović L, Ljubojević D, Lukač D, Lević J, Popović S, et al. Effect of garlic, black pepper and hot red pepper on productive performances and blood lipid profile of broiler chickens. Europ Poult Sci. 2015;79. https://doi.org/10.1399/eps.2015.73.
-
7.
Nasimi P, Vahdati A, Tabandeh MR, Khatamsaz S. Cytoprotective and anti-apoptotic effects of Satureja khuzestanica essential oil against busulfan-mediated sperm damage and seminiferous tubules destruction in adult male mice. Andrologia. 2016;48(1):74-81. [PubMed ID: 26011020]. https://doi.org/10.1111/and.12421.
-
8.
Masouri B, Salari S, Khosravinia H, Vakili Tabatabaei SS, Mohammadabadi T. Effects of dietary Satureja khuzistanica essential oils and α-tocopherol on productive performance, organ weights, blood lipid constituents and antioxidative potential in heat stressed broiler chicks. Eur Poult Sci. 2015. https://doi.org/10.1399/eps.2015.96.
-
9.
Khosravinia H. Effects of Satureja khuzistanica essential oils in drinking water on mortality, production performance, water intake, and organ weights in broiler chickens reared under heat stress condition. Int J Biometeorol. 2015;59(11):1711-9. [PubMed ID: 25791163]. [PubMed Central ID: PMC4602056]. https://doi.org/10.1007/s00484-015-0979-9.
-
10.
Hadian J, Hossein Mirjalili M, Reza Kanani M, Salehnia A, Ganjipoor P. Phytochemical and morphological characterization of Satureja khuzistanica Jamzad populations from Iran. Chem Biodivers. 2011;8(5):902-15. [PubMed ID: 21560239]. https://doi.org/10.1002/cbdv.201000249.
-
11.
Ahmadvand H, Tavafi M, Khosrowbeygi A. Effects of Satureja Khozestanica essential oil on hemoglobin A1C, serum urea and creatinine in alloxan-induced type 1 diabetic adult rats. Pars of Jahrom University of Medical Sciences. 2013;11(1):57-62. https://doi.org/10.29252/jmj.11.1.8.
-
12.
Khosravinia H. Hypolipidemic effects of satureja khuzistanica essential oil in broiler chicken are realized through alteration in steroid hormones. Univ Vet Fak Derg. 2015;21:203-9. https://doi.org/10.9775/kvfd.2014.12020.
-
13.
Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875-7. [PubMed ID: 17446889]. https://doi.org/10.1038/nprot.2007.102.
-
14.
National Research Council. Nutrient Requirements of Sheep. 6th ed. Washington, DC: Natl Acad Press; 2007.
-
15.
Qureshi MA, Havenstein GB. A comparison of the immune performance of a 1991 commercial broiler with a 1957 randombred strain when fed "typical" 1957 and 1991 broiler diets. Poult Sci. 1994;73(12):1805-12. [PubMed ID: 7877936]. https://doi.org/10.3382/ps.0731805.
-
16.
Iji PA. The impact of cereal non-starch polysaccharides on intestinal development and function in broiler chickens. Worlds Poult Sci J. 2019;55(4):375-87. https://doi.org/10.1079/wps19990026.
-
17.
Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. 2014;94(2):341-8. [PubMed ID: 24037967]. https://doi.org/10.1002/jsfa.6365.
-
18.
SAS Institute. SAS User's Guide, Release 9.1.3. Cary, North Carolina: SAS Institute; 2005. Available from: https://support.sas.com/documentation/onlinedoc/91pdf/sasdoc_913/genetics_ug_10108.pdf.
-
19.
Jaguezeski AM, Perin G, Bottari NB, Wagner R, Fagundes MB, Schetinger MRC, et al. Addition of curcumin to the diet of dairy sheep improves health, performance and milk quality. JAFST. 2018;246:144-57. https://doi.org/10.1016/j.anifeedsci.2018.10.010.
-
20.
Karangiya VK, Savsani HH, Patil SS, Garg DD, Murthy KS, Ribadiya NK, et al. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet World. 2016;9(3):245-50. [PubMed ID: 27057106]. [PubMed Central ID: PMC4823283]. https://doi.org/10.14202/vetworld.2016.245-250.
-
21.
Elagib A, ElAmin A, Elamin E. Effect of dietary garlic (allium sativum) supplementation as feed additive on broiler performance and blood profile. J Anim Sci Adv. 2013;3(2):58. https://doi.org/10.5455/jasa.20130219104029.
-
22.
Eid KM, Iraqi MM. Effect of garlic powder on growth performance and immune response for newcastle and avian influenza virus diseases in broiler of chickens. 2nd International Conference On Biotechnology Applications In Agriculture (ICBAA). Moshtohor and Hurghada, Egypt. Benha University; 2014. p. 8-12.
-
23.
Lee KW, Everts H, Kappert HJ, Frehner M, Losa R, Beynen AC. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br Poult Sci. 2003;44(3):450-7. [PubMed ID: 12964629]. https://doi.org/10.1080/0007166031000085508.
-
24.
Freitas RD, Fonseca JB, Soares RDTRN, Rostagno HS, Soares PR. Utilization of garlic (Allium sativum L.) as growth promoter of broilers. Braz J Anim Sci. 2001;30(3):761-5. https://doi.org/10.1590/s1516-35982001000300022.
-
25.
Kırkpınar F, Ünlü H, Özdemir G. Effects of oregano and garlic essential oils on performance, carcase, organ and blood characteristics and intestinal microflora of broilers. Livestock Science. 2011;137(1-3):219-25. https://doi.org/10.1016/j.livsci.2010.11.010.
-
26.
Singh J, Sharma M, Singh N, Kaur P, Sethi APS, Sikka SS. Effect of sun dried whole bulb garlic powder on nutrient utilization, blood parameters, duodenum morphology and faecal microbial load in broiler chickens. Indian J Anim Sci. 2017;87(2):195-8.
-
27.
Barbagallo CM, Averna MR, Frada G, Noto D, Cavera G, Notarbartolo A. Lipoprotein profile and high-density lipoproteins: subfractions distribution in centenarians. Gerontology. 1998;44(2):106-10. [PubMed ID: 9523222]. https://doi.org/10.1159/000021992.
-
28.
Hotta M, Nakata R, Katsukawa M, Hori K, Takahashi S, Inoue H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J Lipid Res. 2010;51(1):132-9. [PubMed ID: 19578162]. [PubMed Central ID: PMC2789773]. https://doi.org/10.1194/jlr.M900255-JLR200.
-
29.
Zantop DW. Biochemistries. In: Ritchie BW, Harrison GJ, Harrison LR, editors. Avian Medicine: Principles and Applications. Lake Worth, FL: Wingers Publishing Inc; 1997. p. 115-29.
-
30.
Khan RU, Nikousefat Z, Tufarelli V, Naz S, Javdani M, Laudadio V. Garlic (Allium sativum) supplementation in poultry diets: effect on production and physiology. Worlds Poult Sci J. 2019;68(3):417-24. https://doi.org/10.1017/s0043933912000530.
-
31.
Brenes A, Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim Feed Sci Tech. 2010;158(1-2):1-14. https://doi.org/10.1016/j.anifeedsci.2010.03.007.
-
32.
Hong J, Steiner T, Aufy A, Lien T. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livestock Science. 2012;144(3):253-62. https://doi.org/10.1016/j.livsci.2011.12.008.
-
33.
Amresh G, Reddy GD, Rao Ch V, Singh PN. Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol. 2007;110(3):526-31. [PubMed ID: 17097249]. https://doi.org/10.1016/j.jep.2006.10.009.
-
34.
Afaq S, Rao PVR, Tariq H, Mondal BC. Effect of garlic and chromium picolinate supplementation on production performance, carcass characteristics and immunological parameters of guinea fowls. J Anim Res. 2016;6(1):99. https://doi.org/10.5958/2277-940x.2016.00016.4.
-
35.
Jang IS, Ko YH, Yang HY, Ha JS, Kim JY, Kim JY, et al. Influence of essential oil components on growth performance and the functional activity of the pancreas and small intestine in broiler chickens. Asian-Austral J Anim Sci. 2004;17(3):394-400. https://doi.org/10.5713/ajas.2004.394.
-
36.
Ocak N, Erener G, Burak Ak F, Sungu M, Altop A, Ozmen A. Performance of broilers fed diets supplemented with dry peppermint (Mentha piperita L.) or thyme (Thymus vulgaris L.) leaves as growth promoter source. Czech J Anim Sci. 2008;53(No. 4):169-75. https://doi.org/10.17221/373-cjas.
-
37.
Dieumou FE, Teguia A, Kuiate JR, Tamokou JD, Fonge NB, Dongmo MC. Effects of ginger (Zingiber officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Cali, Colombia: CIPAV Foundation; 2009.
-
38.
Sharifi-Rad J, Sharifi-Rad M, Hoseini-Alfatemi SM, Iriti M, Sharifi-Rad M, Sharifi-Rad M. Composition, cytotoxic and antimicrobial activities of satureja intermedia C.A.Mey essential oil. Int J Mol Sci. 2015;16(8):17812-25. [PubMed ID: 26247936]. [PubMed Central ID: PMC4581223]. https://doi.org/10.3390/ijms160817812.
-
39.
Amadbr A, Zentek K. Effects of a phytogenic feed additive on growth performance, selected blood
criteria and jejunal morphology in broiler chickens. Emirates J Sci Food Agric. 2013;25(7):549. https://doi.org/10.9755/ejfa.v25i7.12364. -
40.
Viveros A, Chamorro S, Pizarro M, Arija I, Centeno C, Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 2011;90(3):566-78. [PubMed ID: 21325227]. https://doi.org/10.3382/ps.2010-00889.