Abstract
Context:
Functions of melanocytes are supported by many growth factors and cytokines derived from epidermal and dermal cells, especially keratinocytes and fibroblasts. In addition to these skin cells, elastin fibers are thought to be important for regulating melanocyte functions in normal and abnormal skin. However, the role of elastin has not been fully investigated. Recently, the research on the role of elastin in melanocyte functions has greatly increased even in human skin, whereas no full review article on this subject has been presented so far. Thus, the author tried to present a complete review article on this subject in animals and humans.Methods:
The author searched the literature on elastin and melanocytes in research databases.Results:
Elastin fibers can affect the normal functions of melanoblasts/melanocytes in animals and humans. Moreover, elastin fibers influence melanocyte functions in abnormal skin. In vitiliginous skin, where melanin and melanoblasts/melanocytes are completely lost, elastin fibers are dramatically reduced. However, elastin fibers are completely restored or even increased in the dermis of repigmented skin after phototherapy and/or skin transplantation. Moreover, elastin fibers penetrate the basement membrane of repigmented skin, suggesting a direct contact between dermal elastin fibers and epidermal melanoblasts/melanocytes.Conclusions:
Elastin/elastin fibers may control the functions of mammalian melanocytes in normal and abnormal skin.Keywords
1. Context
In mammalian skin, pigment-producing cells, melanocytes, are located mainly in the epidermis, dermis, and hair follicles (1). Melanin produced by melanocytes regulates the expression of coat color in animals and protects the skin against harmful stimuli such as ultraviolet (UV) light. In mammals, two types of melanins are produced. One is brown- to black-colored eumelanin with higher molecular weight, and the other is yellow- to orange-colored pheomelanin with lower molecular weight (2). Eumelanin is derived from L-tyrosine, and its synthesis is mainly controlled by tyrosinase (TYR), TYR-related protein-1 (TRP1/TYRP1, DHICA-oxidase), and -2 (TRP2/TYRP2, dopachrome tautomerase (DCT)) (3). On the other hand, pheomelanin is derived from cysteinyldopa produced by the reaction of L-tyrosine-derived dopaquinone with L-cysteine (2).
Melanin is accumulated in small organelles called melanosomes. Melanosome maturation is categorized into four stages, namely, stages I (initiation of intraluminal matrix formation but no TYR and melanin), II (completed intraluminal matrix formation but no TYR and melanin), III (initiation of melanin depositions on the intraluminal matrix by TYR), and IV (mature melanosome with full melanin depositions). Mammalian melanocytes have all stages of melanosomes, whereas melanoblasts have only stages I and II melanosomes. Melanocyte differentiation is mainly controlled by TRP1, TRP2/DCT, and TYR (1, 3).
Melanoblasts originating from embryonic neural crest cells invade the epidermis from the dermis and differentiate into melanocytes. Differentiated epidermal melanocytes migrate into hair follicles and constitute hair bulb melanocytes, where mature stage IV melanosomes are transferred to surrounding keratinocytes, giving rise to melanized hair (4-8). Melanized hair contributes to the expression of the coat color in animals.
In mice, epidermal melanocytes are observed only one month after birth except for glabrous skin such as ears, nose, foot pads, and tail. Thus, melanocytes in the adult cutaneous skin are located in the hair follicles but not the epidermis (1). Melanin present in hair bulb melanocytes and hair shafts seems to protect skin from harmful UV. By contrast, in humans, melanocytes are present in the epidermis and hair follicles throughout their life. Therefore, melanin in epidermal melanocytes seems to protect the skin against UV (9). Moreover, the epidermis’ anatomical characteristics greatly differ between mice and humans. The inter-follicular epidermis of mice is generally flat, whereas that of humans is wavy and rich in rete ridges (9). Although the differences in the functions between the rete ridge epidermis and inter-rete ridge epidermis are not known, melanoblasts/melanocytes located in the rete ridge epidermis are more numerous than in the inter-rete ridge epidermis (10), suggesting the possibility that melanocytes present in the rete ridge epidermis contribute more greatly to the protection against UV.
The development of mammalian melanocytes is regulated by numerous factors derived from surrounding tissue environments, especially keratinocytes (11-13), fibroblasts (14-16), endothelial cells (17), and blood vessels (18). They produce and release numerous mitogens and melanogens towards melanocytes. The interaction between melanocytes and the surrounding tissue environments is important for regulating the function of mammalian melanocytes. Recently, dermal fibers such as collagen and elastin fibers have been paid particular attention in addition to these factors (19-22). Collagen fibers are major dermal components, and their precursors, collagen molecules, have triple-helix conformation, produce long thin fibrils or two-dimensional reticular, or connect with other extracellular matrix elements. Collagen is synthesized as long precursors, procollagens, in fibroblasts and released from them. Collagen fibers control mechanical stability, elasticity, and strength (23). By contrast, elastin fibers consist of two elements, microfibrils, and matrix elastin. Fibrillins and microfibril-associated glycoproteins constitute microfibrils (24). Elastin is synthesized in fibroblasts as a linear polypeptide, tropoelastin, and released into extracellular space. Tropoelastin undergoes extensive cross-linking by lysyl oxidase in the growing network (24). Elastin possesses a unique repeating sequence, valine-glycine-valine-alanine-proline-glycine (VGVAPG). This hexapeptide is repeated multiple times in human elastin molecules (24).
In addition to keratinocytes and fibroblasts, elastin fibers are thought to be important for regulating melanocyte functions in normal and abnormal skin. However, the role of elastin has not been fully investigated. Recently, the research on the role of elastin in melanocyte functions has greatly increased even in human skin, whereas no full review article on this subject has been presented so far. Thus, the author tried to present a complete review of this subject in animals and humans. Moreover, the mechanism of regulating mammalian melanocyte function by elastin/elastin fibers is also discussed.
2. Evidence Acquisition
We performed a literature search in databases such as Web of Science, Current Contents, and PubMed using keywords including “melanocyte,” “melanoblast,” “melanocyte development,” “melanocyte differentiation,” “melanocyte function,” “elastin,” “elastin fibers,” “collagen,” “collagen fibers,” “elastin fibers and melanocytes,” “vitiligo,” “vitiligo and melanoblast/melanocytes,” “vitiligo and elastin,” and “vitiligo repigmentation.” These databases were searched up to February 2023.
3. Results
3.1. Mammalian Melanocyte Development
The skin has three kinds of cells: Keratinocytes, melanocytes, and fibroblasts. Mammalian melanocytes are developed from melanoblasts originating from embryonic neural crest cells (1). Epidermal invaginations (hair germ), including keratinocytes and melanoblasts/melanocytes, produce hair follicles. Hair bulbs at the bottom of the hair follicles contain keratinocytes, melanocytes, and fibroblasts (special fibroblasts called dermal papillae cells). The hair bulb melanocytes secrete mature melanosomes into surrounding keratinocytes, producing melanized hair (1, 4-8).
Embryonic murine melanoblasts initiate the formation of stages I and II melanosomes by the enzymatic activities of TRP1 and TRP2/DCT. These melanoblasts can be detected by histochemistry using a combined dopa-premelanin reaction (1, 6) or immunohistochemistry using an anti-HMB-45 antibody (25). Melanocytes are induced to differentiate from melanoblasts by TYR activity and to initiate the formation of stages III and IV melanosomes. These melanocytes can be detected by histochemistry using the dopa reaction (1, 6) or immunohistochemistry using anti-TYR antibody (3).
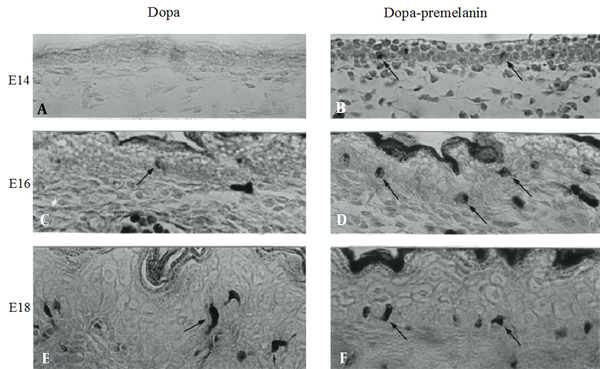
In black mice of strain C57BL/10JHir, no dopa-positive melanocytes were found in the embryonic epidermis at 14 days of gestation (E14) (Figure 1A). By contrast, melanoblasts positive to the combined dopa-premelanin reaction already appeared at E14 (Figure 1B). On the other hand, a small number of melanocytes (Figure 1C) were first found in the E16 epidermis, whereas many melanoblasts were found (Figure 1D). In the E18 epidermis, melanocytes increased in number (Figure 1E), and the combined number of melanoblasts and melanocytes also increased (Figure 1F).
Histochemical sections of the dorsal skin of E14, E16, and E18 days of embryonic mice of strain C57BL/10JHir. A, C, E, Dopa-positive cells and cells positive to the combined dopa-premelanin reaction; B, D, F, Are revealed at the basal layer of the epidermis. Magnification × 400
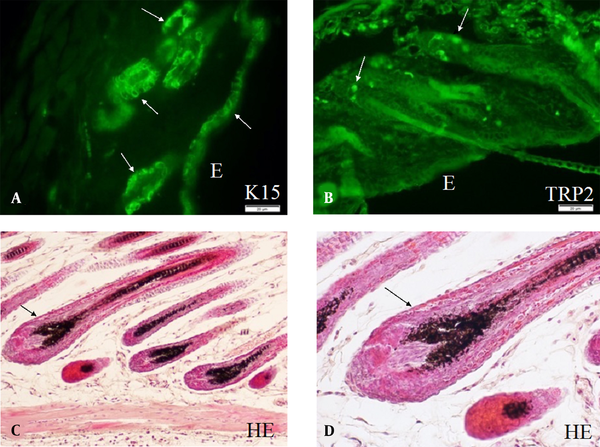
Murine skin cells are derived from many stem cell populations. Skin stem cells play the most important role in maintaining the homeostasis of the skin cells through proliferation and differentiation. Skin stem cells are developed along with embryonic skin development and are finally localized in stem cell niches. Keratinocytes are derived from keratinocyte stem cells (26), and melanoblasts/melanocytes are derived from melanocyte stem cells (27). Keratinocyte stem cells are located both in the epidermis and hair follicles and are served as the niche for melanocyte stem cells in the bulge area of hair follicles (hair bulge). Numerous murine keratinocyte stem cells can be observed in the epidermis, hair follicles, and hair bulges, as shown by immunohistochemistry using anti-K15 antibodies (Figure 2A). On the other hand, a small number of murine melanocyte stem cells can be observed by immunohistochemistry using anti-TRP2 antibody. These melanocyte stem cells are located mainly in the hair bulges (Figure 2B). Differentiating keratinocytes and melanoblasts/melanocytes in the hair bulges can migrate into regenerating hair follicles (anagen hair follicles). Thus, the new anagen hair bulbs have numerous melanocytes with full pigmentation. These melanocytes can be easily detected without dopa reaction (Figure 2C and D).
A, Keratinocyte stem cells; B, Melanocyte stem cells; and C, Differentiated melanocytes observed in the skin of mice of strain C57BL/10JHir. Keratinocyte stem cells (arrows) detected by anti-K15 antibody are observed in E, the epidermis; and A, Hair bulges of the first telogen phase of 24-day-old mice; B, By contrast, melanocyte stem cells (arrows) detected by anti-TRP2/DCT antibodies are observed in the hair bulges of the first telogen phase of 24-day-old mice; C, D, Hematoxylin and eosin staining of the skin of the second anagen hair follicles of 35-day-old mice; C, Pigmented melanocytes are observed in the hair bulbs; D, Higher magnification of the second anagen hair follicles of 35-day-old mice shows pigmented melanocytes and mature melanosomes transported to surrounding keratinocytes. Magnification A, B, × 400; and C, D, × 800
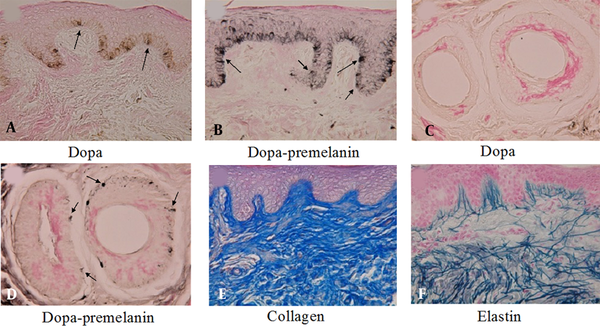
By contrast, in the human epidermis, dopa-positive melanocytes (Figure 3A) and melanoblasts (Figure 3B) positive to the combined dopa-premelanin reaction can be observed even in the epidermis of adult skin. Moreover, even in the absence of dopa-positive melanocytes in the outer root sheath of hair follicles (Figure 3C), numerous melanoblasts (Figure 3D) reactive to the combined dopa-premelanin reaction are observed. These results suggest that human melanoblasts are present in both the epidermis and the outer root sheath of hair follicles, even in adult skin.
In the dermis of the buttocks of a 20-year-old lady, collagen (Figure 3E) and elastin (Figure 3F) fibers are well developed. Moreover, some of the elastin fibers penetrate the basement membrane of the epidermis (Figure 3F), suggesting that the contact between epidermal melanoblasts/melanocytes and elastin fibers is performed in normal skin.
A, B, Histochemical sections of the thumb’s skin derived from a 53-year-old woman. A, The dopa reaction revealed many melanocytes (arrows) with dendrites in the basal layer of the epidermis; and B, The combined dopa-premelanin reaction revealed melanocytes and melanoblasts; C, D, Melanoblasts (short arrows) are lightly stained, while melanocytes (long arrows) are darkly stained. Histochemical sections of the face skin of a 9-year-old boy; C, There is no dopa-positive melanocyte in the outer root sheath of hair follicles; D, By contrast, the combined dopa-premelanin reaction revealed many melanoblasts (arrows) in the outer root sheath of hair follicles; In the dermis of the buttocks of a 20-year-old lady, well-developed E, collagen; and F, elastin fibers are observed. Some of the elastin fibers penetrated the basement membrane of the epidermis. Magnification × 400
3.2. Mammalian Melanocytes and Elastin/Elastin Fibers in Normal Skin
The interaction between normal mammalian melanocytes and elastin/elastin fibers has not been intensively studied. Classical studies using electron microscopy show that the dermal melanocytes in the skin of monkeys (Cynomolgus macaques) interact with dermal elastin fibers (28). Chang et al. (29) observed that the development of epidermal melanoblasts/melanocytes in the skin of C57BL/6J mice correlated well with elastin-binding protein expression. Moreover, elastin-derived peptides (VGVAPG) stimulate differentiation, dendritogenesis, and melanosome maturation in cultured melanocytes (29). Recently, Hirobe and Enami (20) reported that in human melanocytes, elastin peptides in the presence of ferrous ferric chloride (FFC (30) regulate the oxidation and reduction status in cells, stimulate the functions of murine and human keratinocytes, fibroblasts, and melanocytes in vivo and in vitro) increase TYR activity, melanin content, melanocyte proliferation, and epidermal melanin pigmentation. These studies suggest that the interaction between mammalian epidermal melanocytes/melanoblasts and dermal elastin/elastin fibers may be important for regulating melanocyte functions (Table 1).
Role of Elastin and Elastin Fibers in the Control of Melanocyte Function in the Normal and Abnormal Skin
| No. | Species | Date a | Ref. | Skin | Research | Observation | Result |
|---|---|---|---|---|---|---|---|
| 1 | Monkey | 1985 | (28) | Normal | Observation | EM | Dermal M interacts with EF |
| 2 | Human | 1985 | (28) | DM | Observation | LM/EM | Dermal M interacts with EF |
| 3 | Human | 2004 | (31) | Melanoma | Culture + EP | BC/EM | MMP-2↑, melanoma invasion↑, contact with EF |
| 4 | Mouse | 2008 | (29) | Normal | Embryonic skin | LM | EBP expression in Mb/M↑ as age advanced |
| 5 | Mouse | 2008 | (29) | Normal | Culture + EP | LM/EM | M Diff↑, dendritogenesis↑, melanin↑ |
| 6 | Human | 2008 | (32) | VLS | Observation | LM/EM | EF↓ |
| 7 | Human | 2010 | (33) | ELT | Observation | LM | EF↓ |
| 8 | Human | 2012 | (34) | Melanoma | Culture + EP | BC | Melanin↑, TYR↑, mRNA of ETBR and c-kit↑ |
| 9 | Human | 2013 | (35) | AEGCG | Observation | LM | ROS↑, CD3+-, CD4+-T cells↑, ELT↑, MMP-2↑, EF↓, M↓ |
| 10 | Human | 2014 | (36) | ELT | Observation | LM | EF↓ |
| 11 | Human | 2019 | (37) | Normal | Observation | LM | EF↓, Melanin in aged man↓ |
| 12 | Human | 2020 | (19) | VIT | Observation | LM | EF↓, M/Mb = 0 |
| 13 | Human | 2022 | (20) | Normal | FFC + EP | LM | M↑, EF↑ |
| 14 | Human | 2022 | (21) | VIT | MEL/NB-UVB and/or ST | LM | M↑, EF↑ |
| 15 | Human | 2022 | (38) | VIT | SW+UCB-MSCCS+EP+FFC | LM | M↑, EF↑ |
3.3. Human Melanocytes and Elastin/Elastin Fibers in Abnormal Skin
Recently, the relationship between human melanocytes in the abnormal skin and elastin/elastin fibers has been paid much attention, especially in dermal melanocytosis (DM), melanoma, aged skin, vitiligoid lichen sclerosus (VLS), annular elastolytic giant cell granuloma (AEGCG), elastophagocytosis (ELT), and vitiligo (VIT). Vitiligo is the most frequent of these diseases, and the affected lesions are caused by the absence of melanin and melanocytes (19, 21, 28, 31-36, 38). Although the mechanism of vitiligo development and repigmentation after phototherapy and/or skin transplantation is still unclear (42, 43), melanocyte death by infiltrating CD8+ T lymphocytes is one of the potent hypotheses (44). The other hypothesis is that melanocyte death is due to the deficiency of growth factors and cytokines released from the surrounding tissue environments (45).
The classical study by Ono et al. (28) revealed that in DM (abnormal proliferation of dermal melanocytes), dendrites of dermal melanocytes are aligned along the long axis of elastin fibers. Ntayi et al. (31) reported that elastin fibers are contacted with melanoma, and one of the proteases related to an invasion of tumor cells, matrix metalloproteinase-2 (MMP-2), is increased in melanoma cells cultured on elastin peptides-coated dishes. Tian et al. (34) also reported that elastin peptides increase melanin content, TYR activity, and mRNA levels of endothelin receptor B and c-kit in A375 human melanoma cells in culture. Endothelin and c-kit are mitogenic factors towards melanocytes (1). Langton et al. (37) reported that the contents of elastin fiber and epidermal melanin in the forearm of the aged African-American volunteers were greatly reduced compared to the young ones. Similar observations have been reported by Hirobe and Enami: Elastin fibers are lower in the aged volunteers (20) than in the younger ones (19, 21, 38). Langton et al. (37) and Hirobe et al. (19, 21, 38) present an assumption that the disruption of elastin fiber organization is detrimental to melanocyte function.
Roles of elastin fibers have been studied for the development and repigmentation of VIT. Hirobe et al. reported that elastin fibers but not collagen fibers are dramatically decreased in the vitiliginous skin (19), and the decrease of elastin fibers is completely restored after repigmentation by phototherapy using monochromatic excimer light/narrow-band UVB (MEL/NB-UVB) and/or skin (mini-graft) transplantation (21). Moreover, the repigmentation is greatly induced by the combined treatment of stem cell culture supernatant, collagen peptides, elastin peptides, FFC, mini-graft transplantation, and phototherapy using MEL/NB-UVB (38). In the repigmented skin, elastin fibers become thicker and denser and reach the basal layer of the epidermis through the basement membrane (21).
These observations suggest direct contact between melanoblasts/melanocytes/melanoma and elastin fibers. Thus, the downregulation of elastin/elastin fibers may induce VIT, whereas their upregulation may induce VIT repigmentation. This hypothesis seems to be supported by the assumption that the destruction of elastin fibers induces immune infiltrate (CD8+ T cells) followed by vitiligo development, and the construction of elastin fibers inhibits immune infiltrate followed by repigmentation in the VIT skin because the decrease and increase of elastin fibers in VIT skin well correlate with the melanocyte loss and redifferentiation, respectively. The proliferation and differentiation of melanocytes may be closely related to elastin fiber development in the normal and abnormal dermis. The regeneration of elastin fibers may inhibit the infiltration of CD8+ T cells, whereas the degradation of elastin fibers may facilitate their infiltration.
The mechanism leading to elastin fiber degradation in abnormal circumstances, such as AEGCG (35) and ELT (36), includes degradative enzymatic processes. It has been shown that reactive oxygen species (ROS) and other free radicals (FR) are increased in VIT (35). Also, ROS and FR may increase the expression of MMPs, reducing elastin fibers. The reduced elastic fibers may then induce inflammation with granuloma formation. The inflammation then induces ELT (36), like AEGCG, developing on the vitiliginous skin (35). Moreover, VLS, an autoimmune disease with a depigmentation area, changes elastin fibers. This disease is associated with VIT in many cases (32). Therefore, VLS is similar to VIT clinically, but VLS possesses the characteristics of VIT and LS histologically.
The inflammation in LS is thought to be triggered against melanocytes in a similar tendency as VIT (32). In LS lesions, elastin fibers are also degraded (33). The loss of elastin fibers in LS lesions may be due to enzymatic digestion mediated by inflammatory cells and/or ELT, which is a kind of phagocytosis of normal and abnormal elastin fibers by histiocytes and multinucleated giant cells (33). The normal elastin fibers seem to be phagocytosed by macrophages, followed by the reduction of elastin fibers. The reduction may activate macrophages, resulting in ELT. Taken together, elastin fiber reduction in VIT seems to be due to enzymatic degradation such as MMP-2 and/or ELT (35). However, these hypotheses remain to be investigated in detail in a future study.
4. Conclusions
Elastin and elastin fibers are thought to be involved in regulating the functions of mammalian melanocytes in normal and abnormal skin because in the dermis of aged skin, VLS, ELT, AEGCG, VIT, elastin fibers, and melanocytes are greatly reduced or lost. In addition, in the DM, melanoma, and repigmented skin after phototherapy and/or skin transplantation, elastin fibers are completely recovered, and melanocyte redifferentiation and melanogenesis are greatly induced. The interaction between elastin fibers and epidermal and dermal melanocytes has been observed in the skin of animals and humans. Moreover, elastin peptides stimulate the differentiation of murine and human melanoblasts/melanocytes. Overall, elastin/elastin fibers seemingly regulate melanocyte differentiation and survival in normal and abnormal circumstances such as skin diseases.
References
-
1.
Hirobe T. How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Res. 2011;24(3):462-78. [PubMed ID: 21375698]. https://doi.org/10.1111/j.1755-148X.2011.00845.x.
-
2.
Wakamatsu K, Munyard K, Oddie C, Ito S. Photobleached Oxidative Degradation of Melanins: Chemical Characterization of Melanins Present in Alpaca Fiber. Photochem Photobiol. 2021;97(6):1493-7. [PubMed ID: 34435360]. https://doi.org/10.1111/php.13511.
-
3.
Yamaguchi Y, Hearing VJ. Melanocytes and their diseases. Cold Spring Harb Perspect Med. 2014;4(5). [PubMed ID: 24789876]. [PubMed Central ID: PMC3996377]. https://doi.org/10.1101/cshperspect.a017046.
-
4.
Dilshat R, Vu HN, Steingrimsson E. Epigenetic regulation during melanocyte development and homeostasis. Exp Dermatol. 2021;30(8):1033-50. [PubMed ID: 34003523]. https://doi.org/10.1111/exd.14391.
-
5.
Bonnamour G, Soret R, Pilon N. Dhh-expressing Schwann cell precursors contribute to skin and cochlear melanocytes, but not to vestibular melanocytes. Pigment Cell Melanoma Res. 2021;34(3):648-54. [PubMed ID: 33089656]. https://doi.org/10.1111/pcmr.12938.
-
6.
Hirobe T. Structure and function of melanocytes: microscopic morphology and cell biology of mouse melanocytes in the epidermis and hair follicle. Histol Histopathol. 1995;10(1):223-37.
-
7.
Michalak-Micka K, Buchler VL, Zapiorkowska-Blumer N, Biedermann T, Klar AS. Characterization of a melanocyte progenitor population in human interfollicular epidermis. Cell Rep. 2022;38(9):110419. [PubMed ID: 35235792]. https://doi.org/10.1016/j.celrep.2022.110419.
-
8.
Hsu YC, Fuchs E. Building and Maintaining the Skin. Cold Spring Harb Perspect Biol. 2022;14(7). [PubMed ID: 34607830]. [PubMed Central ID: PMC8977401]. https://doi.org/10.1101/cshperspect.a040840.
-
9.
Hirobe T, Enami H. Histochemical study of the distribution of epidermal melanoblasts and melanocytes in Asian human skin. Skin Res Technol. 2019;25(3):299-304. [PubMed ID: 30387525]. https://doi.org/10.1111/srt.12649.
-
10.
Hirobe T, Enami H, Nakayama A. The human melanocyte and melanoblast populations per unit area of epidermis in the rete ridge are greater than in the inter-rete ridge. Int J Cosmet Sci. 2021;43(2):211-7. [PubMed ID: 33296514]. https://doi.org/10.1111/ics.12682.
-
11.
Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125(15):2915-23. [PubMed ID: 9655813]. https://doi.org/10.1242/dev.125.15.2915.
-
12.
Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17(2):96-110. [PubMed ID: 15016298]. https://doi.org/10.1111/j.1600-0749.2003.00126.x.
-
13.
Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment Cell Res. 2005;18(1):2-12. [PubMed ID: 15649147]. https://doi.org/10.1111/j.1600-0749.2004.00198.x.
-
14.
Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112 ( Pt 12):1843-53. [PubMed ID: 10341204]. https://doi.org/10.1242/jcs.112.12.1843.
-
15.
Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193-9. [PubMed ID: 19449448]. [PubMed Central ID: PMC2793097]. https://doi.org/10.1002/biof.29.
-
16.
Hirobe T, Hasegawa K, Furuya R, Fujiwara R, Sato K. Effects of fibroblast-derived factors on the proliferation and differentiation of human melanocytes in culture. J Dermatol Sci. 2013;71(1):45-57. [PubMed ID: 23726358]. https://doi.org/10.1016/j.jdermsci.2013.03.012.
-
17.
Kim M, Shibata T, Kwon S, Park TJ, Kang HY. Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Sci Rep. 2018;8(1):4235. [PubMed ID: 29523807]. [PubMed Central ID: PMC5844989]. https://doi.org/10.1038/s41598-018-22608-y.
-
18.
Shibata T, Kajiya K, Sato K, Yoon J, Kang HY. 3D microvascular analysis reveals irregularly branching blood vessels in the hyperpigmented skin of solar lentigo. Pigment Cell Melanoma Res. 2018;31(6):725-7. [PubMed ID: 30117278]. https://doi.org/10.1111/pcmr.12731.
-
19.
Hirobe T, Enami H, Nakayama A. Elastin fiber but not collagen fiber is decreased dramatically in the dermis of vitiligo patients. Int J Dermatol. 2020;59(10):e369-72. [PubMed ID: 32346870]. https://doi.org/10.1111/ijd.14896.
-
20.
Hirobe T, Enami H. Elastin Peptides with Ferrous Ferric Chloride Activate Human Melanocytes and Elastin Fibers. J Skin Stem Cell. 2022;9(2). https://doi.org/10.5812/jssc-127254.
-
21.
Hirobe T, Enami H. Reduced Elastin Fibers and Melanocyte Loss in Vitiliginous Skin Are Restored after Repigmentation by Phototherapy and/or Autologous Minigraft Transplantation. Int J Mol Sci. 2022;23(23). [PubMed ID: 36499690]. [PubMed Central ID: PMC9739647]. https://doi.org/10.3390/ijms232315361.
-
22.
Shenoy M, Abdul NS, Qamar Z, Bahri BMA, Al Ghalayini K, Kakti A. Collagen Structure, Synthesis, and Its Applications: A Systematic Review. Cureus. 2022;14(5). https://doi.org/10.7759/cureus.24856.
-
23.
Romero-Ortuno R, Kenny RA, McManus R. Collagens and elastin genetic variations and their potential role in aging-related diseases and longevity in humans. Exp Gerontol. 2020;129:110781. [PubMed ID: 31740390]. https://doi.org/10.1016/j.exger.2019.110781.
-
24.
Rauscher S, Pomes R. The liquid structure of elastin. Elife. 2017;6. [PubMed ID: 29120326]. [PubMed Central ID: PMC5703643]. https://doi.org/10.7554/eLife.26526.
-
25.
Gleason BC, Crum CP, Murphy GF. Expression patterns of MITF during human cutaneous embryogenesis: evidence for bulge epithelial expression and persistence of dermal melanoblasts. J Cutan Pathol. 2008;35(7):615-22. [PubMed ID: 18312434]. [PubMed Central ID: PMC2935278]. https://doi.org/10.1111/j.1600-0560.2007.00881.x.
-
26.
Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci U S A. 1993;90(15):7391-5. [PubMed ID: 8346261]. [PubMed Central ID: PMC47143]. https://doi.org/10.1073/pnas.90.15.7391.
-
27.
Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416(6883):854-60. [PubMed ID: 11976685]. https://doi.org/10.1038/416854a.
-
28.
Ono T, Mah K, Hu F. Dermal melanocytes and elastic fibers. J Cutan Pathol. 1985;12(6):468-75. [PubMed ID: 4078109]. https://doi.org/10.1111/j.1600-0560.1985.tb00445.x.
-
29.
Chang CH, Kawa Y, Tsai RK, Shieh JH, Lee JW, Watabe H, et al. Melanocyte precursors express elastin binding protein and elastin-derived peptide (VGVAPG) stimulates their melanogenesis and dendrite formation. J Dermatol Sci. 2008;51(3):158-70. [PubMed ID: 18487037]. https://doi.org/10.1016/j.jdermsci.2008.03.010.
-
30.
Hirobe T. Iron and skin health: iron stimulates skin function. In: Preedy VR, editor. Handbook of diet, nutrition and the skin. Wageningen, Netherlands: Wageningen Academic Publishers; 2012. p. 196-214. https://doi.org/10.3920/978-90-8686-729-5_12.
-
31.
Ntayi C, Labrousse AL, Debret R, Birembaut P, Bellon G, Antonicelli F, et al. Elastin-derived peptides upregulate matrix metalloproteinase-2-mediated melanoma cell invasion through elastin-binding protein. J Invest Dermatol. 2004;122(2):256-65. [PubMed ID: 15009703]. https://doi.org/10.1046/j.0022-202X.2004.22228.x.
-
32.
Attili VR, Attili SK. Vitiligoid lichen sclerosus: a reappraisal. Indian J Dermatol Venereol Leprol. 2008;74(2):118-21. [PubMed ID: 18388368]. https://doi.org/10.4103/0378-6323.39693.
-
33.
Abbas O, Chatrath V, Goldberg LJ. Elastophagocytosis in extragenital lichen sclerosus. J Cutan Pathol. 2010;37(10):1032-7. [PubMed ID: 20602660]. https://doi.org/10.1111/j.1600-0560.2010.01575.x.
-
34.
Tian S, He P, Zhang J, Chen Z. Effect of kappa elastin on melanogenesis in A375 human melanoma cells and its related mechanism. Chin Med J. 2012;125(22):4088-92.
-
35.
Watabe D, Akasaka T. Annular elastolytic giant cell granuloma developing on lesions of vitiligo. Int J Dermatol. 2013;52(11):1458-60. [PubMed ID: 23113612]. https://doi.org/10.1111/j.1365-4632.2011.05301.x.
-
36.
El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70(5):934-44. [PubMed ID: 24447829]. https://doi.org/10.1016/j.jaad.2013.12.012.
-
37.
Langton AK, Alessi S, Hann M, Chien AL, Kang S, Griffiths CEM, et al. Aging in Skin of Color: Disruption to Elastic Fiber Organization Is Detrimental to Skin's Biomechanical Function. J Invest Dermatol. 2019;139(4):779-88. [PubMed ID: 30404021]. https://doi.org/10.1016/j.jid.2018.10.026.
-
38.
Hirobe T, Enami H. Mesenchymal Stem Cell-Derived Factors Stimulate the Differentiation of Melanocytes in the Vitiliginous Skin in Combination With Phototherapy. J Cosmet Sci. 2022;73:391-403.
-
39.
Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, et al. Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol. 2013;22(4):245-50. [PubMed ID: 23425123]. https://doi.org/10.1111/exd.12103.
-
40.
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74-84. [PubMed ID: 25596811]. https://doi.org/10.1016/S0140-6736(14)60763-7.
-
41.
Marchioro HZ, Silva de Castro CC, Fava VM, Sakiyama PH, Dellatorre G, Miot HA. Update on the pathogenesis of vitiligo. An Bras Dermatol. 2022;97(4):478-90. [PubMed ID: 35643735]. [PubMed Central ID: PMC9263675]. https://doi.org/10.1016/j.abd.2021.09.008.
-
42.
Wu X, Yang Y, Xiang L, Zhang C. The fate of melanocyte: Mechanisms of cell death in vitiligo. Pigment Cell Melanoma Res. 2021;34(2):256-67. [PubMed ID: 33346939]. https://doi.org/10.1111/pcmr.12955.
-
43.
Taieb A. Vitiligo as an inflammatory skin disorder: a therapeutic perspective. Pigment Cell Melanoma Res. 2012;25(1):9-13. [PubMed ID: 22099450]. https://doi.org/10.1111/j.1755-148X.2011.00939.x.
-
44.
Riding RL, Harris JE. The Role of Memory CD8(+) T Cells in Vitiligo. J Immunol. 2019;203(1):11-9. [PubMed ID: 31209143]. [PubMed Central ID: PMC6709670]. https://doi.org/10.4049/jimmunol.1900027.
-
45.
Biswas KB, Takahashi A, Mizutani Y, Takayama S, Ishitsuka A, Yang L, et al. GPNMB is expressed in human epidermal keratinocytes but disappears in the vitiligo lesional skin. Sci Rep. 2020;10(1):4930. [PubMed ID: 32188902]. [PubMed Central ID: PMC7080742]. https://doi.org/10.1038/s41598-020-61931-1.