Abstract
Background:
Psoriasis is a chronic, recurrent, genetic skin disease without any definite cure. Unfortunately, studies on psoriasis are limited in our country and few studies have been done regarding its risk factors and time of occurrence.Objectives:
The current study aimed to assess risk factors of psoriasis recurrence through proportional rates model.Patients and Methods:
The current study was conducted at the Department of Dermatology, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran, from March 2006 through April 2014. A total of 160 patients with confirmed diagnosis of psoriasis were recruited and risk factors for the recurrence of psoriasis were identified and evaluated by applying the proportional rates model.Results:
The results of this study indicated that infection, stress, family history, blood calcium level, smoking, and number of white blood cells were risk factors for recurrence of psoriasis. Moreover, 56.3% of patients did not have any leading-to-hospitalization relapse after enrolling in the study with one relapse episode reported in 23%, two in 10.6%, three in 4.4%, and four or more relapse episodes in 5.6% of participants.Conclusions:
It is essential to identify people with psoriasis. Another important point is to inform patients of the causes of this disease to decrease the risk of recurrence. In addition, it is recommended to educate patients about reduction or cessation of smoking as a part of psoriasis treatment.Keywords
1. Background
Psoriasis is an autoimmune disease with genetic basis and different rate of penetration (1). Psoriasis affects 1% to 6% of the population and equally affects both sexes as well as different races. The disease might occur at all ages and might present at birth. The mean age at presentation is 37.8 years (2). Typically, it is not a life-threatening disease but sometimes it causes inability due to joints involvement. Prevalence is reported to range from 1% to 3% in different parts of the world and it is estimated that between one million and three million people are affected in Iran. Age of onset is often second to fourth decades of life and might begin in younger ages in those with a family history of disease.
Psoriasis might be aggravated by beta-hemolytic streptococcal infections, abrupt cessation of steroids, stress, pregnancy, and taking certain treatments such as Lithium or systemic corticosteroids. Most studies have been performed on chronic plaque psoriasis, which is the most common form of disease (2, 3). Unfortunately, accurate statistics on the prevalence of psoriasis is not available in Iran and studies on this issue are limited. Determining recurrence rate as well as identifying factors that affect psoriasis recurrence would be helpful in prolonging recovery period and reducing disease recurrence.
In the current study, we decided to identify risk factors associated with recurrence of psoriasis with use of proportional rates model. The most popular and simplest model in context of recurrent data analysis is Anderson-Gill model or AG model, which was introduced in 1982. In this model, the sequences of events that occur for each experimental unit are considered as a counting process with independent development. AG model is used when researchers only want to estimate the overall rate of an event at different times.
2. Objectives
The aim of this study was to investigate the factors influencing recurrence of patients with psoriasis by using proportional rate model (PRM).
3. Patients and Methods
The current study was approved by the Ethical Committee of the Medical Sciences Faculty of Tarbiat Modares University. The research was conducted as a longitudinal study at Dermatology Department of Imam Khomeini Hospital, Tehran, Iran, from March 2006 through April 2014. We investigated patients’ records from the documentation center of Imam Khomeini Hospital. After a preliminary assessment, a total of 160 patients were identified and followed up. Only patients who had relapses leading to hospitalization and were regularly referred to the hospital were included. The current status of patients was assessed through making a phone call to them or their family.
The primary outcome measure was the frequency of the recurrence. Firstly, the number of relapses leading to hospitalization from diagnosis through the end of the study was investigated and then its precipitating factors were analyzed. Basic characteristics including age at diagnosis, sex, marital status, family history, year of the study entry, blood calcium levels, place of residence, comorbid diseases, and white blood cells count were obtained from the hospital records. Some additional data such as data on stress, sunburn, temperature changes, diet, smoking, infection, previous history of psoriasis, and occupation were also obtained through phone calls.
In the current study, PRM was applied to identify risk factors for recurrence of psoriasis (Equation 1).
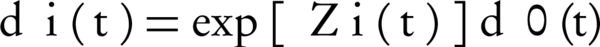
In which dµ0(t) is base rate function, Z(t) is vector of covariates in t, and β is vector of regression coefficients. In this study, we used R software (coxph function) to analysis data.
4. Results
A total of 160 patients, including 89 males (55.6%), who had been diagnosed with psoriasis were recruited. Mean age of the patients by sex is shown in Table 1, and the frequency of relapses leading to hospitalization among patients is shown in Table 2.
Among the 160 patients, 47 (37.29%) were single and 113 (62.70%) were married; moreover, 57 patients (36%) had no history of psoriasis. The mean duration of disease in 103 patients was 6.4 ± 6.75 years (median, 5 years) and the highest duration was 40 years. A history of psoriasis in family members or close relatives was reported by 41 patients (25.6%) while 119 patients (74.3%) mentioned no history of disease in family members. Housewives, students, and employees constituted 50, 15, and seven out of 72 females in this study. Thus, much of the variation in occupation was related to men. Patients were classified as normocalcemic (2.2 to 2.55 mmol/L), hypocalcemic (< 2.2 mmol/L), and hypercalcemic (> 2.55 mmol/L) regarding their blood calcium levels (Table 3). Moreover, patients were divided according to their white blood cell (WBC) count into two groups of normal WBCs (< 10 × 109 cells/L) and leukocytosis (> 10 × 109 cells/L ) (Table 4) (4). The results of the fitting PRM showed no significant association between psoriasis recurrence and sex, year of study, previous history of psoriasis, age at study entry, place of residence, occupation, year of study entry, comorbid diseases, and diet (P > 0.05). According to the coefficients (Table 5), infection and marital status had association with psoriasis relapse. Thus, the rate of relapse was 38% less in married than was in single patients. Furthermore, infection increased rate of recurrence by 7%. Smoking, family history, and temperature changes had increased recurrence rate of disease by two, three, and 1.5 times, respectively. Other factor that affected the incidence of relapse was stress (P < 0.05). Relapse rate was 82% more frequent in patients with stress in comparison with those without stress.
| Sex | Age at Entrance, y | Median, y |
|---|---|---|
| Female | 47 ± 17.22 | 48.5 |
| Male | 40.87 ± 16.92 | 38 |
| Total | 43.49 ± 17.29 | 40 |
| Recurrence | Results |
|---|---|
| None | 56.3 (90) |
| Once | 23.1 (37) |
| Twice | 10.6 (17) |
| Three Times or More | 8.38 (16) |
| Patients’ Blood Calcium Status | Results |
|---|---|
| Hypocalcemia | 56 (35) |
| Normal | 88 (55) |
| Hypercalcemia | 16 (10) |
| Total | 160 (100) |
| White Blood Cell Count | Results |
|---|---|
| Normal | 104 (65) |
| Leukocytosis | 56 (35) |
Results of the Fitting Proportional Rates Model for Recurrence Rate in Patients With Psoriasis
| Risk Factors | Relative Rate (95% CI) | P Value |
|---|---|---|
| Infection | ||
| No (base) | ||
| Yes | 1.07 (1.04-3.78) | 0.043 |
| Marital status | ||
| Single (base) | ||
| Married | 0.62 (0.43-1.66) | 0.022 |
| Smoking | ||
| No (base) | ||
| Yes | 2.01 (1.54-2.46) | 0.044 |
| Stress | ||
| No (base) | ||
| Yes | 1.82 (0.79-2.27) | 0.04 |
| Temperature changes | ||
| No (base) | ||
| Yes | 2.95 (2.34-8.45) | 0.001 |
| Family history | ||
| No (base) | ||
| Yes | 1.47 (0.99-2.28) | 0.03 |
| White blood cell | ||
| Normal (base) | ||
| Leukocytosis | 1.24 (0.79-2.23) | 0.035 |
| Blood Calcium Level | ||
| Normal (2.2-2.55 mmol/L) | ||
| Hypocalcemia (< 2.2 mmol/L) | 1.19 (1.04-2.03) | 0.011 |
| Hypercalcemia (> 2.55 mmol/L) | 0.82 (0.12-1.43) | 0.16 |
5. Discussion
This study investigated the effects of demographic and metabolic factors on psoriasis relapse rate. After using the fitting PRM, we determined that the risk of recurrence was significantly reduced in married participants. Although it seemed that this effect might be due to younger age in single patients, the effect of age was not significant according to our analysis; hence, the effect of marital status on the rate of relapse would be independent of age. Moreover, those with severe disease would be more likely to remain single. Another factor that showed a significant effect in the fitted model was the WBC count that had been classified into normal levels and leukocytosis. Patients with higher WBC count had higher risk of recurrence leading to hospitalization than those with normal WBC count did. Patients with infection-related relapses had higher WBC count; therefore, with the elimination of the infectious agent, disease recurrence was also reduced. Other significant factors in relapsing were smoking and temperature changes. Some evidence showed that smoking is as an independent risk factor in the onset of psoriasis lesions. In some studies, the heaviness of smoking was associated with clinical severity of psoriasis while smoking duration had not such an association. In addition, smoking reduces the rate of recovery and response to treatment in patients with psoriasis. Reduction of tobacco use is shown to reduce relapses frequency and severity of psoriasis (4-9). In a study by Al'Abadie et al. (10), significant correlation between smoking and risk of recurrence was observed, indicating that smoking doubles the risk of relapse. In a study on 200 patients with psoriasis in England, smoking habits before disease onset were studied and a significant correlation between smoking and risk of psoriasis was reported (11). In a case-control study conducted in Italy, family history (OR = 7; 95% CI,5.28-9.82), stress (OR = 1.7; 95% CI,0.14-3.36), and infection (OR = 7.8; 95% CI,4.75-8.9) had a significant correlation with recurrence of psoriasis, which was consistent with the results of our study. In another study in London, infection was identified as a major factor in recurrence of psoriasis (12) and in another case-control study on 310 patients with psoriasis, smoking and alcohol (OR = 2.29; 95% CI,1.24-4.81), family history (OR = 33.96; 95% CI, 28.61-39.54), and changes in environmental conditions (OR = 8.34; 95% CI,5.12-10.27) were identified as risk factors for recurrence of psoriasis (13). In a study by Neumann et al. (14), cigarette smoking (OR = 1.31; 95% CI, 1.29-1.34) was considered as an important risk factor for the recurrence of psoriasis. The results of a study by Farshchian showed that in contrast to age, sex, and type of psoriasis, smoking and alcohol were risk factors for recurrence of psoriasis (15), which was in agreement with the results of the present study. Many times, analyzing recurrent events and specifying the correlations between events that had happened to each person are very complicated or impossible. Therefore, using models that are based on the severity function in these cases does not seem right because we should know the correlation structure and include it into the model or we should assume that this correlated structure would be in the model with covariates; however, this assumption is not acceptable in most cases. In contrast, Observations of each person are dependent, most of statistical models need to impose this correlation but this model unlike other models don’t. Therefore, when correlation structure is unknown, this model is recommended for the formulation of the occurrence of events. Finally, the data collected for this study were derived from the contents of their records and this information is not reliable for statistical analysis; therefore, it is better to use them in making assumptions for more detailed studies.
Acknowledgements
References
-
1.
Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8(1):1-12. [PubMed ID: 17093502]. https://doi.org/10.1038/sj.gene.6364351.
-
2.
Griffiths C, Camp R. Psoriasis. In: Burns T, Breathnach S, editors. RooksTextbook of Dermatology. 17th ed. Massachusetts: Blackwell; 2004. p. 35-69.
-
3.
Habif T. Psoriasis and other Papulosquamous Diseases. In: Habif T, editor. ClinicalDermatology. 4th ed. New York: Mosby; 2004. p. 209-45.
-
4.
Bradley PJ, Griffith C, Barker J. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-71.
-
5.
Behnam SM, Behnam SE, Koo JY. Smoking and psoriasis. Skinmed. 2005;4(3):174-6. [PubMed ID: 15891254].
-
6.
Daniell HW. Smoker's wrinkles. A study in the epidemiology of "crow's feet". Ann Intern Med. 1971;75(6):873-80. [PubMed ID: 5134897].
-
7.
Cai J, Schaubel DE. Analysis of recurrent event data. Amsterdam: Elsevier; 2004.
-
8.
Bo K, Thoresen M, Dalgard F. Smokers report more psoriasis, but not atopic dermatitis or hand eczema: results from a Norwegian population survey among adults. Dermatology. 2008;216(1):40-5. [PubMed ID: 18032898]. https://doi.org/10.1159/000109357.
-
9.
Sherwin MA, Gastwirth CM. Detrimental effects of cigarette smoking on lower extremity wound healing. J Foot Surg. 1990;29(1):84-7. [PubMed ID: 2181017].
-
10.
Al'Abadie MS, Kent GG, Gawkrodger DJ. The relationship between stress and the onset and exacerbation of psoriasis and other skin conditions. Br J Dermatol. 1994;130(2):199-203. [PubMed ID: 8123572].
-
11.
Just M, Ribera M, Monso E, Lorenzo JC, Ferrandiz C. Effect of smoking on skin elastic fibres: morphometric and immunohistochemical analysis. Br J Dermatol. 2007;156(1):85-91. [PubMed ID: 17199572]. https://doi.org/10.1111/j.1365-2133.2006.07575.x.
-
12.
Zheng GY, Wei SC, Shi TL, Li YX. Association between alcohol, smoking and HLA-DQA1*0201 genotype in psoriasis. Acta Biochim Biophys Sin (Shanghai). 2004;36(9):597-602. [PubMed ID: 15346196].
-
13.
Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25(6):606-15. [PubMed ID: 18021899]. https://doi.org/10.1016/j.clindermatol.2007.08.015.
-
14.
Neumann AL, Shin DB, Wang X. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829-35.
-
15.
Farshchian MA. Psoriasis and risk factors of the metabolic syndrome: a case-control research study. Skin Beauty.